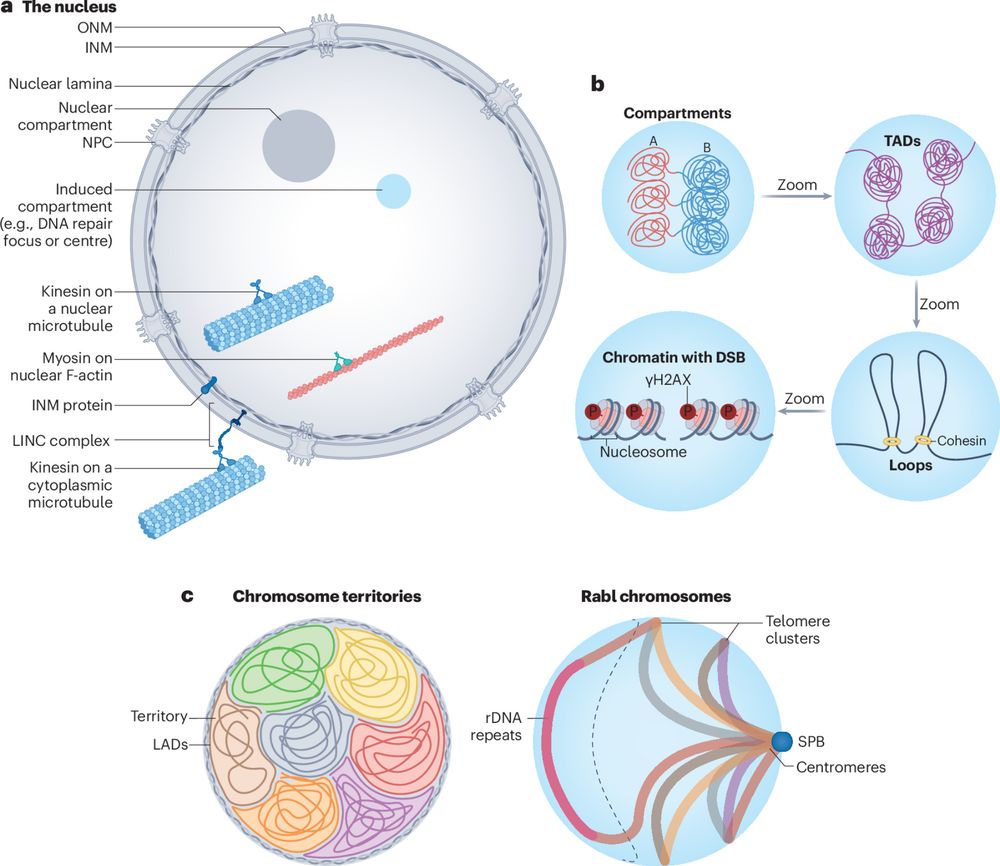
Happy that @drmelaniew.bsky.social and I could play a small role in this lovely work led by @ssegurabayona.bsky.social. Please do give the preprint a look.
11.09.2025 08:35 — 👍 0 🔁 0 💬 0 📌 0


📢Deadline extended!
Our 2nd Spatial Genome Organisation Conference deadline has been extended to 25 Sep, dont miss out on joining our fantastic line up of speakers, alongside Karim, Matthias and Irene in what is set to be a fantastic meeting this October! #SGO25
Final slots ➡️https://bit.ly/3JJzYR4
02.09.2025 14:34 — 👍 0 🔁 3 💬 1 📌 1
Lovely work Pragathi!
06.08.2025 00:56 — 👍 0 🔁 0 💬 0 📌 0
A shout out to lab member @szmyd-radoslaw.bsky.social for his recent recognition by @sydney.edu.au. Well earned Radek!
06.08.2025 00:55 — 👍 6 🔁 0 💬 0 📌 0

A person seated in a laboratory setting with shelves, containers, and scientific equipment in the background.
We’re proud of our researcher Dr Radek Szmyd who has received a 2025 University of Sydney Faculty of Medicine and Health Mid-Career Research Outstanding Publication Award - for Radek’s extraordinary work to understand why cancer cells die in different ways following radiotherapy.
17.07.2025 06:59 — 👍 1 🔁 1 💬 0 📌 1
DNA replication and Cryo-EM star Jacob Lewis is visiting @cmri.bsky.social today to present seminar. If you happen to be in Westmead, talk is 12:30 pm local time. See you there.
20.06.2025 01:54 — 👍 0 🔁 0 💬 0 📌 0

Hunter Meeting 2025
Excited to speak at the Hunter Meeting next week, alongside @cmri.bsky.social colleagues Pragathi Masamsetti (postdoc with Patrick Tam, proud Cesare lab alum) and Pengyi Yang (Head, Computational Systems Biology Unit). Looking forward to great science and good company.
www.huntermeeting.org.au
27.05.2025 01:56 — 👍 3 🔁 1 💬 0 📌 0
Home | acpc
If you happen to be the the Oz Single Cell Conference this week in Sydney, check out @scientistkate.bsky.social's talk on Friday.
Kate is a joint postdoc w/@piptaberlay.bsky.social and will present her work on 3D Genome architecture and replication stress.
www.ozsinglecell.com
19.05.2025 23:26 — 👍 3 🔁 1 💬 0 📌 0
ACCM 2025! The Cesare lab will be there in force. Come join us in Melbourne for 3 days of amazing science.
15.05.2025 12:36 — 👍 0 🔁 0 💬 0 📌 0

I'm super excited to announce that registrations are now open for the 19th Australian Cell Cycle, DNA Repair and Telomere Workshop. Awesome international speaker line-up, with plenty of locals being invited! Book now to secure your earlybird rate. www.australiancellcycle.org
15.05.2025 05:48 — 👍 17 🔁 9 💬 1 📌 3
Fantastic opportunity in Melbourne.
14.05.2025 11:55 — 👍 3 🔁 1 💬 0 📌 0
Hosting Lee Wong from @monashuniversity.bsky.social tomorrow to present her seminar: "Onco-histone mutations in paediatric high-grade glioma: molecular mechanisms and implications for therapy." If you are around @cmri.bsky.social tomorrow at 12:30, come by for a great talk.
08.05.2025 01:32 — 👍 3 🔁 0 💬 0 📌 0
@genomestability.bsky.social (Andrew Deans) will be @cmri.bsky.social tomorrow to present seminar at 12:30; "The Multifaceted Role of FANCM enzyme in DNA Repair and Cancer Therapy". If you are in the area, please do come by for a great talk.
10.04.2025 05:23 — 👍 6 🔁 1 💬 0 📌 0
Sorry Sam and @jenniferlovesecoli.bsky.social, I am also unaware of papers/studies exploring nuclear F-actin in c. elegans. Happy to be updated if anyone knows otherwise.
21.03.2025 00:29 — 👍 3 🔁 0 💬 1 📌 0
*basketball... 🤦♂️
21.03.2025 00:15 — 👍 0 🔁 0 💬 0 📌 0
My and @telomerase-cmri.bsky.social’s annual attempt to bring a little bit of college baseketball culture down under.
Go Tar Heels!
20.03.2025 22:17 — 👍 10 🔁 1 💬 1 📌 0
26/Finally, the data are consistent with t-loops suppressing ATM activation by the chromosome end.
T-loops opening in a regulated and shelterin-dependent manner to signal cellular stress suggests a broader role for these chromosome end structures than previously appreciated.
17.03.2025 10:32 — 👍 2 🔁 0 💬 1 📌 0
25/We predict this represents a non-canonical telomere-dependent tumour suppressive activity outside the canonical ageing-dependent proliferative barriers imposed by telomere erosion.
Additionally, we found the protective functions of the TRF2 basic domain are mitosis specific.
17.03.2025 10:32 — 👍 1 🔁 0 💬 1 📌 0
24/What does this mean?
We now understand the pathway of MAD-telomere deprotection that contributes to 1) cell death during mitotic arrest, and 2) p53-dependent cell cycle arrest when cells escape an elongated mitosis.
17.03.2025 10:32 — 👍 1 🔁 1 💬 1 📌 0
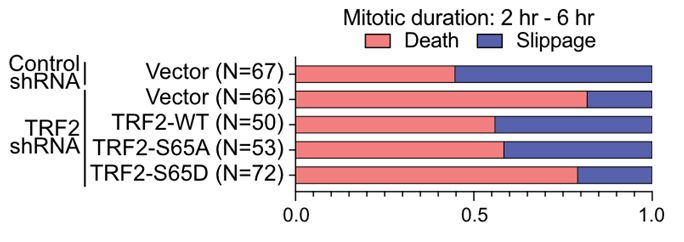
23/Live imaging from both labs revealed that TRF2 mutants that suppress MAD-telomere deprotection also reduce the prevalence of mitotic arrest driven lethality. While TRF2 mutants that promote MAD-telomere deprotection enhanced mitotic death or expedited time of mitotic arrest to lethality.
17.03.2025 10:32 — 👍 0 🔁 0 💬 1 📌 0
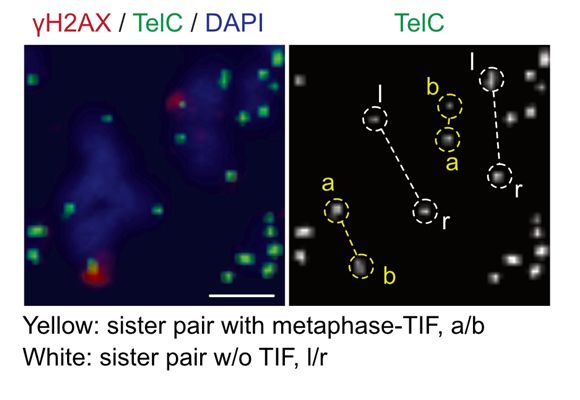
22/Of note, telomeres do not rapidly truncate in MAD-telomere deprotection like they do when the TRF2 basic domain is deleted. Thus, while the TRF2 basic domain is modified to enable BTR-dependent t-loop opening, it does not allow t-loop junction cleavage, consistent with a highly regulated pathway.
17.03.2025 10:32 — 👍 0 🔁 0 💬 1 📌 0
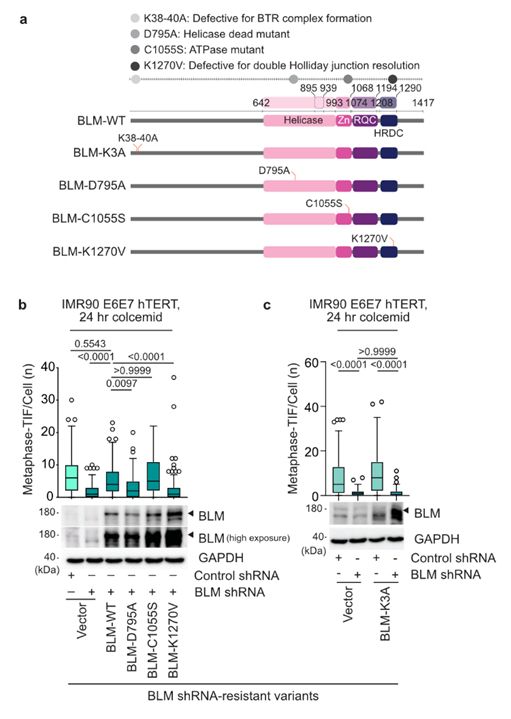
21/Diana’s molecular biology was consistent with the BTR complex being requisite for MAD-telomere deprotection following TRF2 modification.
This suggested that t-loops junctions are in a double Holliday Junction configuration during mitotic arrest before resolution by BTR.
17.03.2025 10:32 — 👍 0 🔁 0 💬 1 📌 0
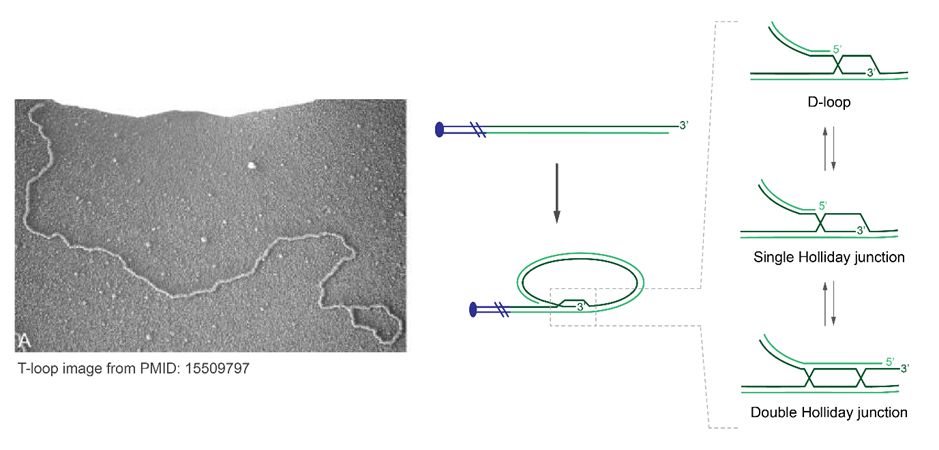
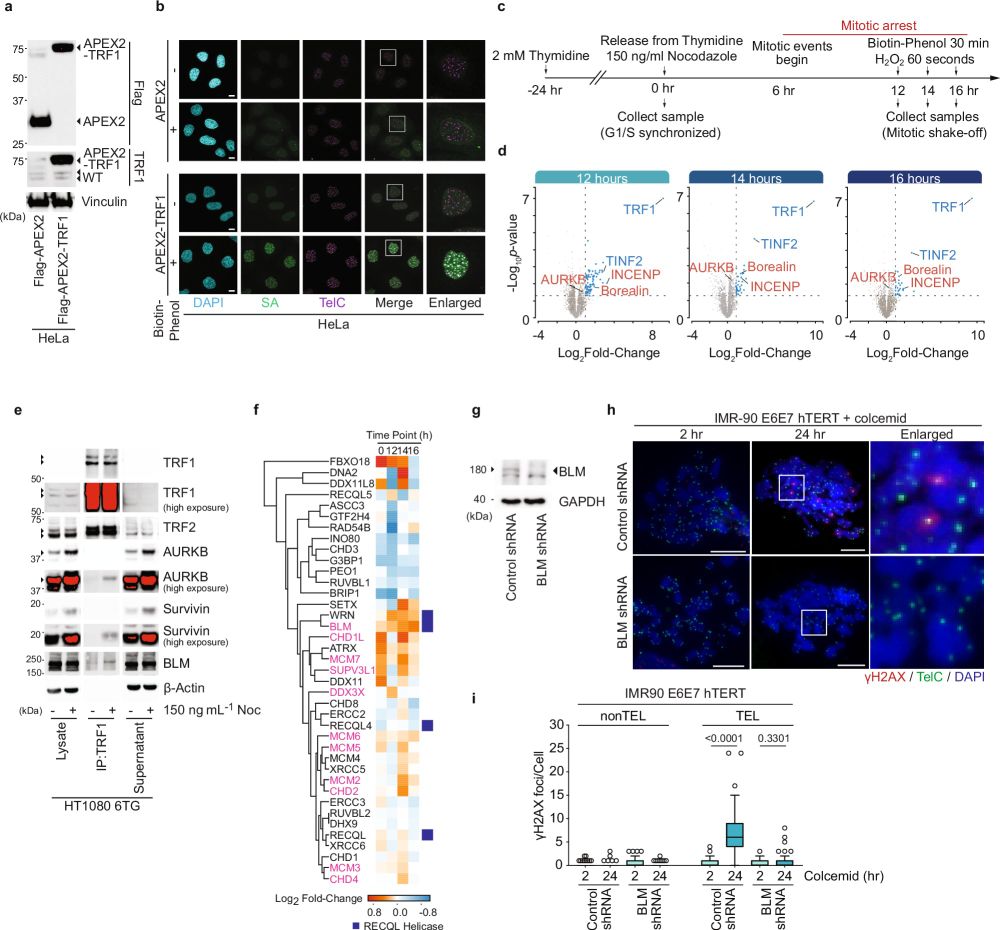
20/How does BLM fit into the story?
BLM does many things, including double Holliday Junction dissolution (dHJ) via the BTR complex. T-loops are typically drawn with strand-invasion promoting a displacement loop. However, this is conjecture, and the t-loop junction identify is unknown.
17.03.2025 10:32 — 👍 0 🔁 0 💬 1 📌 0
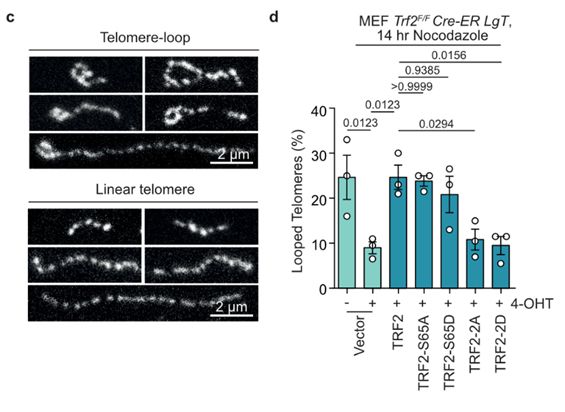
19/Ronnie's Airyscan imaging of telomere macromolecular structure following in situ trioxsalen cross-linking and chromatin spreading suggested TRF2 basic domain phosphorylation promoted the transition from looped to linear telomeres during mitotic arrest, coinciding with telomere DDR induction.
17.03.2025 10:32 — 👍 0 🔁 1 💬 1 📌 0
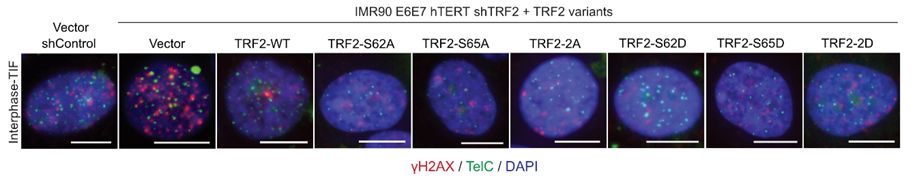
18/Critically, both labs showed that all TRF2 S62 and/or S65 phospo-null or phospho-mimetic mutants retained interphase telomere protection, indicating these residues function specifically in mitotic telomere protection. Diana & Makoto also showed that TRF1 and TRF2 mutants localized to telomeres.
17.03.2025 10:32 — 👍 1 🔁 0 💬 1 📌 0
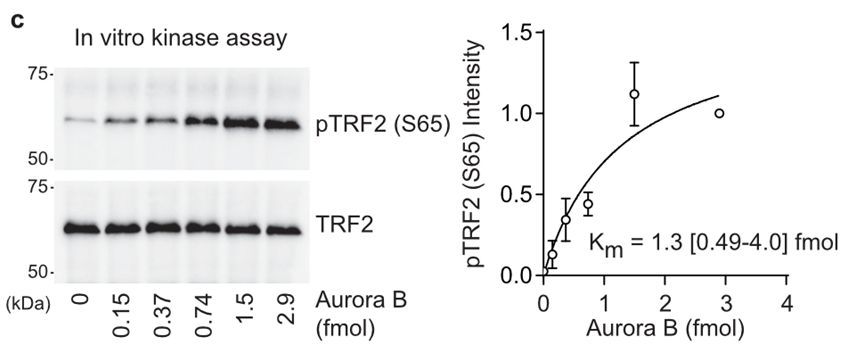
17/Sam created a phosphospecific antibody against pTRF2-S65 and his biochemistry confirmed this residue was phosphorylated specifically during mitotic arrest in an AURKB-dependent fashion.
17.03.2025 10:32 — 👍 0 🔁 0 💬 1 📌 0
Molecular Biologist (aemonten.github.io) 丨Chief Editor @ CSH Protocols (cshprotocols.cshlp.org) 丨Head of the Integrity in Publishing Group at CSHL Press 丨Chair "Molecular Biosystems Conference" (molbiosystems.com) 丨(Oxford) Comma King 丨Central Dogma Police
The preeminent Australian conference on the organisation and expression of the genome
Investigating kinase-dependent regulation of DNA replication and repair
Postdoc in the @karlsederlab.bsky.social at The Salk Institute for Biological Studies
We explore how cells stick together and fall apart..focussing on how cell-cell adhesion, apoptotic extrusion and tissue mechanics regulate epithelial homeostasis 🧫🔬
@AlphaYap’s lab at IMB, Brisbane
Deputy Director at WEHI, Melbourne Australia.
Lab head studying epigenetic control, in the context of X inactivation, genomic imprinting, SMCHD1, Prader Willi Syndrome, FSHD.
Mum, wife, beach lover.
Trends in Cell Biology is a leading reviews journal published by Cell Press covering the latest advances in cell biology. Editor Ilaria Carnevale, PhD.
At Fusion Conferences, we believe that bringing like-minded professionals, academics, and researchers together is an opportunity to make a real impact.
https://www.fusion-conferences.com/
IFOM is a centre focusing on the study of the molecular mechanisms underlying tumors formation and development.
ifom.eu
Official account of the Telomeres and Telomere Transcription in Cancer and Aging lab, led by Claus M. Azzalin, at GIMM.
https://gimm.pt/lab/claus-maria-azzalin-lab/
Official updates from Georgia U.S. Senator Jon Ossoff's office
Dad, husband, President, citizen. barackobama.com
Professor and Chair, Department of Pharmacology
University of Colorado Anschutz Medical Campus
Cancer biologist, metastasis researcher interested in tumor cell plasticity
Colorado and outdoor enthusiast 🏔️🏔️☀️
Geneticist and cell biologist at the University of Southern California | DNA repair | heterochromatin | recombination | nuclear dynamics | nuclear architecture | genome stability.