
Merlin–YAP signaling: emerging mechanisms, functions, and therapeutic approaches
The Hippo signaling pathway has a critical role in regulating tissue growth, development, and tumor suppression. Mutations in NF2, which encodes the tumor suppressor Merlin, disrupt Hippo signaling, causing aberrant activation of YAP/TAZ and contributing to diseases, such as cancer and developmental disorders. Recent studies have identified novel mechanisms by which biomolecular condensation and phosphoinositides regulate Merlin function in Hippo signaling. Furthermore, NF2 deficiency sensitizes cells to ferroptosis, a form of regulated cell death characterized by iron-dependent lipid peroxidation. In this review, I highlight the emerging roles of Merlin–YAP signaling in physiology and disease, focusing on its regulation through biomolecular condensates, its contribution to development and ferroptosis, and its implications for therapeutic interventions.
Merlin–YAP signaling: emerging mechanisms, functions, and therapeutic approaches
22.10.2025 12:03 — 👍 0 🔁 0 💬 0 📌 0

Roles of lysosomal small-molecule transporters in metabolism and signaling
Lysosomes degrade damaged or unwanted cell/tissue components and recycle their building blocks through small-molecule transporters of the lysosomal membrane. They also act as signaling hubs that sense and signal internal cues, such as amino acids, to coordinate cell responses. Recently, the activity of several lysosomal metabolite transporters has been elucidated, bringing new insights into lysosomal functions. Cell biological and structural studies of lysosomal transporters have also highlighted their roles in recruiting signaling complexes to lysosomes and delineated how their substrates gate such hybrid transporter/receptor, or ‘transceptor’, function. In this review, we summarize recent progress in our understanding of lysosomal transporters, with a focus on the export of lysosomal degradation intermediates, the existence of lysosomal amino acid shuttles that regulate the redox state and pH of the lysosomal lumen, and the role of lysosomal transceptors in nutrient and immune signaling.
Roles of lysosomal small-molecule transporters in metabolism and signaling
18.10.2025 12:02 — 👍 2 🔁 1 💬 0 📌 0

The promiscuous ribosomal P-stalk: a new functional portrait
The canonical role of the ribosome is to translate the genetic code into functional proteins. Recent discoveries, however, redefine the eukaryotic ribosome, as a regulatory hub, that senses cellular cues and transmits signals to downstream pathways. The P-stalk, an integral component of the ribosomal GTPase-associated center, once viewed as translational supporter, is now emerging as a key regulatory ribosomal module. It has recently been recognized as an activator of the integrated stress response, reshaping the Gcn1/Gcn20→Gcn2 axis into the new Gcn1/Gcn20/P-stalk→Gcn2 order. The P-stalk's structural plasticity allows also the ribosome to rewire gene expression in response to cellular demands, including cytokine response. In this review, an updated functional portrait of the P-stalk is presented, encompassing both ribosome-dependent and -independent activities.
The promiscuous ribosomal P-stalk: a new functional portrait
08.10.2025 12:02 — 👍 2 🔁 0 💬 0 📌 0

Optimality as a framework for understanding developmental robustness
Embryo growth, morphogenesis, and patterning are complex processes that coordinate between cellular dynamics, fate specification, and multiscale physical forces. Understanding how robustness in embryo development is achieved despite inherent heterogeneities in gene expression, cell properties, and tissue growth is a fundamental question. Although various feedback between gene expression, signaling, and cell and tissue mechanics have been uncovered to confer robustness on developmental systems, measuring variability and robustness from a quantitative perspective often remains challenging. Furthermore, cell fate plasticity, a key mechanism that can confer robustness, is lacking in many developing tissues. This review highlights how recent technological and conceptual advances in quantitative approaches to biology help to overcome these bottlenecks, with a particular focus on how mechanochemical feedback, or alternatively, selectively tuned control parameters, ensure developmental robustness.
Optimality as a framework for understanding developmental robustness
01.10.2025 12:02 — 👍 1 🔁 1 💬 0 📌 0

Organelle-specific signaling of cGAS–STING
Innate immune sensing through cyclic GMP-AMP synthase (cGAS)–stimulator of interferon genes (STING) surveils cytosolic DNA from invading pathogens or damaged organelles and initiates a spectrum of immune responses. It is well established that upon 2′3′-cyclic GMP-AMP (cGAMP) binding, STING exits the endoplasmic reticulum (ER), traverses the Golgi to trigger interferon programs, and finally reaches lysosomes for signal resolution through degradation, revealing a tightly choreographed itinerary for cytokine-driven immunity. However, emerging studies reveal additional layers of spatiotemporal complexity: ER-resident STING tunes in messenger RNA translation and Ca2+ efflux, Golgi-localized STING functions as a proton channel that initiates H+-dependent autophagy and transcription factor EB-directed programs for organelle homeostasis, and various mechanisms for metabolic remodeling and cell fate determination. This review synthesizes emerging organelle-specific mechanisms of cGAS–STING, delineates their roles in physiology and disease, and discusses how an organelle-centric perspective may inform selective, context-sensitive immunotherapies.
Organelle-specific signaling of cGAS–STING
20.09.2025 12:02 — 👍 5 🔁 1 💬 0 📌 0

Physiological aging in three dimensions
Aging is characterized by progressive structural and functional decline, driven partially by epigenetic alterations. While changes in DNA methylation, histone modifications, and chromatin accessibility are well studied, the role of three-dimensional chromatin organization in aging remains underexplored. Advances in chromosome conformation capture technologies have revealed hierarchical chromatin structures, including compartments, topologically associating domains (TADs), and chromatin loops, which are crucial for gene regulation. Emerging evidence suggests that aging changes these structures, leading to altered gene expression and cellular dysfunction. This review summarizes recent findings on age-associated chromatin reorganization, highlighting its impact on transcription and nuclear architecture. It also compares the roles of 3D chromatin organization in aging and senescence, highlighting shared and distinct features in these biological contexts.
Physiological aging in three dimensions
01.09.2025 12:02 — 👍 2 🔁 0 💬 0 📌 0

The Wnt–NAD+ axis in cancer, aging, and tissue regeneration
The intricate interplay between Wnt signaling and nicotinamide adenine dinucleotide (NAD+) biosynthesis has emerged as a crucial axis that influences aging and tissue regeneration. Wnt signaling, a key regulator of cellular proliferation, differentiation, and tissue homeostasis, intersects with NAD+ metabolism, a cornerstone of cellular energy balance and genomic stability. This relationship is mediated through shared regulatory pathways involving sirtuins, poly(ADP-ribose) polymerases (PARPs), and metabolic enzymes which are sensitive to cellular NAD+ levels. Dysregulation of either pathway is implicated in cancer, age-related decline, and impaired regenerative capacity. This review consolidates current knowledge of the Wnt–NAD+ axis and highlights its cooperative roles in maintaining tissue integrity and combating the effects of aging. Furthermore, it explores therapeutic approaches targeting this axis to restore tissue health and enhance the capacity for repair, thereby offering promising avenues for addressing age-associated pathologies.
The Wnt–NAD+ axis in cancer, aging, and tissue regeneration
30.08.2025 12:02 — 👍 2 🔁 0 💬 0 📌 0

Navigating AKT-ivity across cellular compartments
Phosphoinositide (PIP)-mediated AKT signaling is essential for cellular homeostasis because it orchestrates crucial processes such as metabolism, survival, proliferation, and motility. Dysregulation of this pathway drives various pathologies, particularly cancer. Although cytosolic activation of AKT has been extensively studied, its emerging roles in the nucleus have gained attention over the past decade. The complexities of AKT compartmentalization and associated functional mechanisms remain largely unexplored. At the plasma membrane, AKT activation occurs at specialized microdomains and cell–cell junctions where it influences polarity, adhesion, and migration. In endosomes, PIPs coordinate intracellular trafficking and cytoskeletal organization with AKT signaling. Protein scaffolds refine AKT signal specificity by assembling complexes. In the nucleus, AKT interacts with the p53–PIP signalosome and specific kinases to regulate oncogenesis and chemoresistance. This review explores PIP-driven spatial regulation of AKT across cellular compartments, emphasizing its role in cellular responses and oncogenesis. Understanding AKT compartmentalization mechanisms provides valuable insights into cancer biology and novel therapeutic strategies.
Navigating AKT-ivity across cellular compartments
29.08.2025 12:02 — 👍 3 🔁 0 💬 0 📌 0

Special issue
Death by ribosome — new insights from Anna Constance Vind, Franklin Zhong & Simon Bekker-Jensen on how ribosomal dysfunction can drive cell death.
Read more: http://dlvr.it/TMVfKc
15.08.2025 10:02 — 👍 0 🔁 0 💬 0 📌 0

Special issue
Dynamic rRNA modifications as a source of ribosome heterogeneity — new insights from Ivan Milenkovic & Eva Maria Novoa.
A fresh perspective on how ribosomes diversify function through epitranscriptomic changes.
Read more: http://dlvr.it/TMSZ68
13.08.2025 10:02 — 👍 1 🔁 0 💬 0 📌 0

Special issue
Cell cycle regulation via the ribotoxic stress response — Connor McKenney & Sergi Regot uncover how ribosome damage impacts cell cycle control.
Read more: http://dlvr.it/TMQRqm
11.08.2025 10:02 — 👍 2 🔁 1 💬 0 📌 0
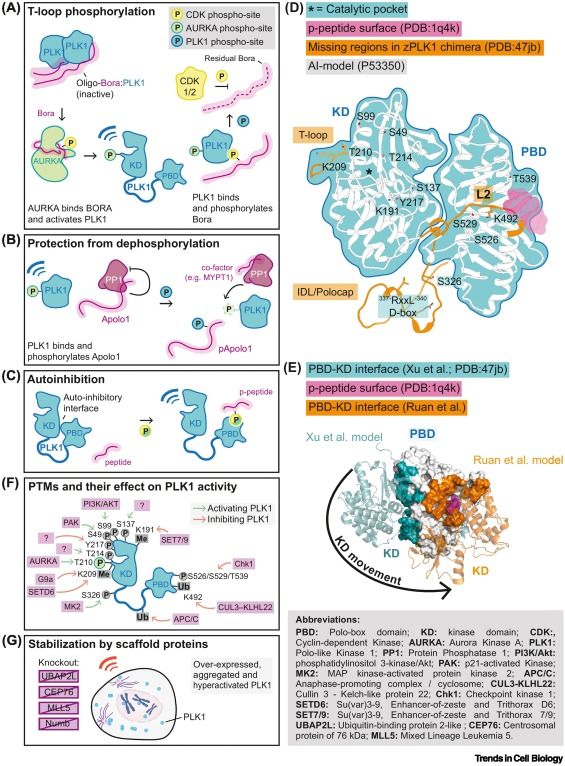
Decoding the language of PLK1 docking motifs and activation mechanisms
Polo-like kinase 1 (PLK1) phosphorylates a plethora of different substrates to regulate key cell cycle processes that include, among others, mitotic entry, chromosome condensation, nuclear envelope breakdown, centrosome maturation, spindle assembly and chromosome biorientation, cytokinesis, and the deposition of the specialized centromere histone CENP-A. Addressing the exact spatial and temporal control of PLK1 activity in these processes and its dynamic interplay with protein phosphatases that counteract mitotic phosphorylation, most notably PP1 and PP2A, has proven especially puzzling. In this review, we focus on the main unknowns in the area of human PLK1 regulation, exploring more specifically an emerging concept that master docking sites, including newly discovered noncanonical motifs, trigger initial local activation of PLK1 that promotes subsequent localized spreading of phosphorylation.
Decoding the language of PLK1 docking motifs and activation mechanisms
09.08.2025 12:03 — 👍 6 🔁 3 💬 0 📌 1

Special issue
MDM4 exon skipping upon dysfunctional ribosome assembly - new findings from Jennifer Jansen & Matthias Dobbelstein reveal a surprising link between ribosome biogenesis and mRNA splicing.
Read more: http://dlvr.it/TMLvmY
07.08.2025 09:57 — 👍 2 🔁 0 💬 0 📌 0

NADH reductive stress drives metabolic reprogramming
Cellular metabolism is intricately regulated by redox signaling, with the NADH/NAD+ couple serving as a central hub. Emerging evidence reveals that NADH reductive stress, marked by NADH accumulation, is not merely a passive byproduct of metabolic dysfunction but an active regulatory signal driving metabolic reprogramming. In this Review, we synthesize recent advances in understanding NADH reductive stress, including its origins, regulatory mechanism, and manipulation. We examine its broad impact on cellular metabolism, its interplay with oxidative and energy stress, and its pathogenic roles in a range of diseases. By integrating these findings, we propose NADH reductive stress as a master regulator for metabolic reprogramming and highlight new avenues for mechanistic exploration and therapeutic intervention.
NADH reductive stress drives metabolic reprogramming
06.08.2025 12:02 — 👍 2 🔁 0 💬 0 📌 0

Special issue
Celebrating 35 years of Trends in Cell Biology!
Explore our special anniversary issue spotlighting ribosomal dynamics and cellular homeostasis.
Dive into the latest insights: http://dlvr.it/TMHXH4
04.08.2025 09:54 — 👍 5 🔁 0 💬 0 📌 0
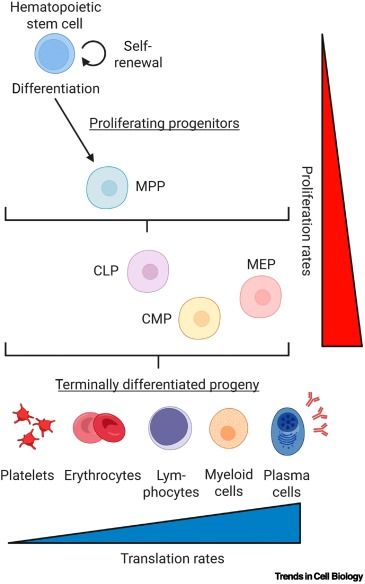
Lessons in longevity from blood stem cells under protein stress
Blood stem cells are among the body’s longest-living cells despite being highly vulnerable to proteotoxic damage, which accelerates their aging. To maintain protein homeostasis (proteostasis), hematopoietic stem cells (HSCs) employ mechanisms such as reduced translation rates, high chaperone activity, autophagy, and selective protein degradation. These strategies mitigate protein misfolding, maintain quiescence, and preserve regenerative potential. Disruptions in proteostasis can lead to the elimination of impaired HSCs through differentiation or apoptosis, ensuring the integrity of the stem cell pool. Due to the systemic impact of the blood on aging and its experimental and clinical accessibility, investigating HSC proteostasis provides insights into longevity and potential therapeutic strategies. This review examines emerging mechanistic links between proteostasis and HSC fate, concluding with unresolved questions and challenges of the current research.
Lessons in longevity from blood stem cells under protein stress
30.07.2025 12:02 — 👍 1 🔁 0 💬 0 📌 0

Science in Mexico: a rising force amid adversity
Despite its economic and population status, Mexico’s scientific output remains under 1% of global production because of low spending on science. Yet, additional challenges, including over-reliance on expensive imported technology, brain drain, and limited private sector investment, further hinder its progress. Nonetheless, significant opportunities exist, such as fostering local biotechnology, enhancing policy continuity, and leveraging new leadership to boost scientific growth. Although focused on Mexico, these insights hold relevance for the broader region of Latin America, a region that shares vast untapped scientific potential.
Science in Mexico: a rising force amid adversity
25.07.2025 12:02 — 👍 1 🔁 0 💬 0 📌 0

Unlocking Latin America´s scientific potential: challenges and opportunities in a globalized world
Latin America shows increasing scientific potential, with a dedicated and creative research community, driven by resilience and adaptability. However, limited funding, restricted access to cutting-edge technology, bureaucratic barriers, and constantly changing scientific policies continue to hinder its full integration into the international scientific ecosystem. Latin American scientists also suffer from limitations in their visibility on the global stage, often leading to exclusion. Despite these challenges, many success cases in the region highlight how strategic actions based on planned and sustained investments, international collaborations, and a relevant scientific policy positively impact scientific progress. Through this path, Latin America may not only overcome existing barriers but also position itself as a fundamental player in the scientific stage.
Unlocking Latin America´s scientific potential: challenges and opportunities in a globalized world
19.07.2025 12:02 — 👍 0 🔁 0 💬 0 📌 0

Communicating science in Latin America: insights, challenges, and future directions
This article reviews public science communication (SC) in Latin America, highlighting advances and challenges. It emphasizes the need to foster inclusive policies, interdisciplinary approaches, and effective evaluation to enhance public engagement, address social inequalities, and foster informed decision-making. Improving this field would strengthen science–society relationships, benefiting both professional communicators and scientific communities.
Communicating science in Latin America: insights, challenges, and future directions
12.07.2025 12:02 — 👍 1 🔁 0 💬 0 📌 0
Official Account for the Lorne Protein Meeting, Australia.
lorneproteins.org
Reviews journal by Cell Press publishes articles covering all aspects of human diseases, diagnostics, therapeutics, and disease prevention. Posts are by the editor, Dr. Aliki Perdikari. Explore more at: https://www.cell.com/trends/molecular-medicine/home
A reviews journal from Cell Press that fosters an appreciation for advances being made on all fronts of genetic research.
Editor: Maria Smit
https://www.cell.com/trends/genetics/home
I post papers on #cancermetabolism and #mitochondrialbiology. I also post about academia, metal music, and mindfulness.
https://www.cecad.uni-koeln.de/research/principal-investigators/prof-dr-christian-frezza/
https://orcid.org/0000-0002-3293-7397
RNA and cancer biologist (opinions, usually strong, are mine)
https://gbiomed.kuleuven.be/english/research/50488876/54502087/research
https://gbiomed.kuleuven.be/english/research/50488876/54502087/Trace
Doing great science by putting people first Neuroscience group protecting axons sciencewithoutanguish.com
Fellow of Churchill College, Cambridge
Coach supporting scientists to get more clarity on their goals and move toward them
Building a community of support for women in mass spectrometry.
Join our LinkedIn group: https://www.linkedin.com/groups/1374586/
Lab of Pekka Katajisto @pekka-katajisto.bsky.social at University of Helsinki and Karolinska Institutet. We study stem cells, aging, metabolism, organelle age and niche interactions https://www.katajisto-lab.com
Associate Professor at MGH Krantz Center for Cancer Research and Harvard Medical School Department of Medicine. Intersection of signaling, metabolism and chemical biology.
Biological physics. Interested in bacteria, cilia, malaria, membranes, microscopy, tweezers, microfluidics and organ on chip.
Membrane transporters, metabolism, drug discovery, systems biology
Studying Immune Cell and #Prostate #Cancer #Metabolism! (She/Her) #NewPI
#Immunometabolism
https://cancerimmunometabolism.com
Tenured PI at FBN. Co-founder of Luminova Biotech. Working on Light and energy replacement. Into anti-aging. My opinions are opinions. Amateur ♟
Scientist bewitched by Tumour metabolism
Group Leader and Lecturer. University of Barcelona @ub.edu & IDIBELL Former @Crick.ac.uk
Biochemist, Veterinary & Olívia's & Jan's dad
Stable isotope-based in vivo metabolomics
https://mmfpd-ub.wixsite.com/amlmetab
Researcher interested in metabolism, nutrition, energy balance. Head of Molecular Physiology of Exercise and Nutrition Department @Leibniz_Dife. Views are my own.
Laboratory of Stem Cell Metabolism at UNIL. Lipid droplets in neural stem cells during development, adulthood and aging. Glial metabolism in health and Alzheimer's disease.
https://wp.unil.ch/knobloch-lab/
studying genetic and environmental contributions to cell fitness in cancer and immunity | biology-engineering-biotechnology | #HPLM | current: investigator @Morgridge_Inst, assistant prof @UW-Madison
Postdoc in Josh Rabinowitz's lab at Princeton University, studying how diet affects cancer therapy











