Thanks #ChembioParis2025 for organising this nice cruise through Paris yesterday evening, following an inspiring conference day! Looking forward to another day of great #chembio today.
08.10.2025 06:20 — 👍 1 🔁 0 💬 0 📌 0
2/2
Thanks to Le Studium, Loire Valley Institute for Advanced Studies, for funding this exciting consortium with great collaborators! Honoured to be involved for proteomics and chemoproteomics projects.
18.09.2025 15:30 — 👍 0 🔁 0 💬 0 📌 0
Back to Greece after a few intense scientific days in Tours, France, in the frame of the « Pharmacological Inhibition of Cathepsin C in Neutrophil-Mediated Inflammatory Diseases » consortium coordinated by Brice Korkmaz at the Centre of studies for Respiratory Pathologies (CEPR). 1/2
18.09.2025 15:30 — 👍 0 🔁 0 💬 1 📌 0
« Médard et al. used a chemoproteomics approach to investigate the off-target effects of tubacin. They identified MBLAC2 as a novel target, and its inhibition or silencing in HEK293 cells increased EV accumulation, suggesting a regulatory role in EV secretion » .
05.09.2025 20:07 — 👍 0 🔁 0 💬 1 📌 0

Stirring Bars are Superstition?
Do you really need to stir your reactions? How do you know?
04.09.2025 19:39 — 👍 59 🔁 11 💬 17 📌 9
go.bsky.app/JKJ1ZHt
04.09.2025 17:38 — 👍 0 🔁 0 💬 0 📌 0
Starter pack: go.bsky.app/2vBi9Ya
Let’s get this list complete !
04.09.2025 17:30 — 👍 1 🔁 0 💬 0 📌 0
#ChemBio #chemoproteomics @kusterlab.bsky.social
04.09.2025 12:43 — 👍 1 🔁 0 💬 0 📌 0
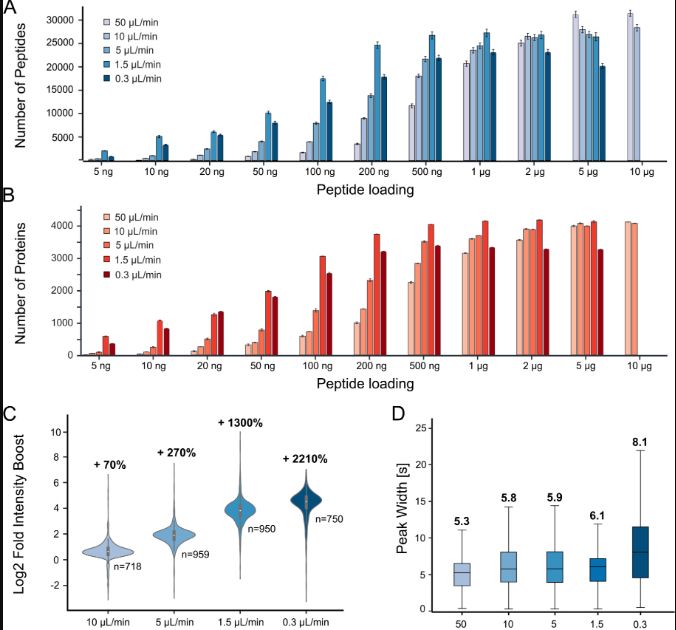
Miniaturisation of the assay allowed by mind-blowing progress in mass-spectrometry equipment and proteomics workflows is a story for another day …
04.09.2025 12:30 — 👍 0 🔁 0 💬 0 📌 0
For the mixing, we have to consider that less is sometimes more. Since there is little chance of achieving ideal complementary of probes with no overlaps, kinases enriched by many probes will dilute the ones enriched by only one in the mass-spec readout, eventually leading to poor quantification.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
Full proteomes and transcriptomics help select biological material with good kinome diversity for our purpose … to a certain extent only, because many kinases have low abundance and because transcripts and protein levels are not always proportional.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
Is kinase X nowhere in the data because no probe can fish it, or because this bugger is not expressed decently in any of the biological material we use? Do we need to test more probes or more kinome-sources? Indeed, all kinases are not expressed everywhere and all the time. Proteomes are dynamic.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
Then comes the testing of individual probes. Once covalently attached to beads, they need to robustly enrich many native kinases of the human (or other) kinome in so-called pulldown experiments. And here comes the challenge and its vicious circle:
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
Or we can also be super pragmatic, avoid synthesis, and limit ourselves to commercial synthetic intermediates and inhibitors which already have an immobilisation handle at the right spot.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
If the final molecules are already quite unselective, that is great for us. If the same molecular scaffold is used for a range of inhibitors relatively selective towards different kinase, great again. And, with those data, we can prioritise the molecules we put synthetic efforts in.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
...“best” as a drug i.e. sub-optimal for us, since they achieve a certain level of selectivity. This criterium of selectivity is generally assessed for all (at best) molecules described against only one or two selected other kinases than the intended target.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
We want a promiscuous probe though … do we have selectivity data in the literature? There will, in general, be panel selectivity data for one or two of the best inhibitors of a class …
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
Looking at X-ray co-crystal structures or docking experiments, we can see where the solvent-pointing part of the molecule is. Can we design a molecule with an immobilisation handle on this spot, that can be easily synthesized? Great. Here we go, this could be an affinity probe.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
The latter are here to achieve a certain level of selectivity towards one kinase or favourable pharmaco-kinetics/dynamics properties, so we want to remove them. Basically, we mentally do the opposite of a medicinal chemist job: we trim down instead of slowly crafting.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
We rely on the structure-activity relationships data tables of each of the many reported molecules to delineate what is the pharmacophore (the part of the molecule we need to absolutely keep for potency) and what are the fine-tuning elements that were introduced by the chemists on the pharmacophore.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
In practice, now. For the selection of a probe for a sub-kinome, we mine the scientific literature and patents for molecules that are designed to hit various kinase families.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
Quite simply, conceptually: we select potent, yet promiscuous pharmacophores that can be immobilised on beads without losing their potency and which are complementary to each other. We make the molecules, prepare the beads, mix them. Bob’s your uncle.
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
Cellzome published a mixture of immobilised kinase inhibitors in 2007, that constituted the first Kinobeads. I joined the Kuster lab in 2011 as a group leader with the clear mission of making Kinobeads even better. How do we do that rationally?
04.09.2025 12:30 — 👍 0 🔁 0 💬 1 📌 0
It is also a good opportunity to reflect on the Kinobeads chemoproteomics assay development over 20 years. Particularly, on the Kinobeads affinity matrix itself.
04.09.2025 12:30 — 👍 1 🔁 1 💬 1 📌 0
The “target landscape of clinical kinase drugs” (2017) has just gotten its 900th citation! There is no shame, I believe, to feel proud of having been part of this effort of the Kuster lab to provide such an - apparently very useful - interactive resource to the scientific community.
04.09.2025 12:30 — 👍 7 🔁 1 💬 2 📌 0
@medard-chemprot.bsky.social in the chembio starter pack for tools (chemoprotemics affinity matrices for kinases and HDACs and target deconvolution/profiling)?
25.08.2025 09:26 — 👍 0 🔁 0 💬 0 📌 0
Chemical biologist. Associate Professor at the University of Leeds. She/her. Views my own.
Professor of Synthetic Electrochemistry - University of Greenwich! Belgian, World Explorer and Medicinal, Organic and Electro chemist ⚡️ . Views are my own.
PhD candidate | Proteomics | Bioinformatics | Precision medicine
@kusterlab
Lab at University of Reading using #archsci #Palaeoproteomics #ZooMS and #Zooarchaeology 🦌🦏🦴🐂 to study past human behaviour; home to project COEXIST. PI Karen Ruebens.
@ChimieParisTech, @psl_univ, @INC_CNRS, i-CleHS, Lab for Inorganic Chemical Biology, PDT, Anticancer, Antimicrobial and Antiparasitic Research, Nuclear Imaging
www.gassergroup.com
Morandi Research Group @ETH Zurich - https://morandi.ethz.ch/
Official home for the #Chemistry community #RealTimeChem (part of #ChemSky). Connecting chemists (on the old place since 2012. Curated by @doctorgalactic.bsky.social.
Join in by sharing chemistry using #RealTimeChem.
Professor at Aarhus University. Total synthesis of natural products. Covalent chemical biology.
Co-founder: Mmimetika Biosciences and Kripthonite Therapeutics
CNRS Research Director - Leader of Targeting of Nucleic Acids' Lab https://icn.univ-cotedazur.fr/tna - President of the French Medicinal Chemistry Society (https://www.sct-asso.fr/) and Director of GDR RNA (https://gdr-rna.cnrs.fr/)
Chemical Biology research group at the University of Arizona. New chemical reactivity for revealing new biology and therapeutic approaches.
Check us out at: taylorresearchgroup.org
The Virtual ChemBioTalks are a series of free annual events in the field of #ChemBio. Free registration required at: cvent.me/G1geWW
Homepage: https://www.universiteitleiden.nl/en/science/led3/chembiotalks
Professor
Drug Design and Pharmacology
University of Copenhagen
Retired Director at Max Planck Institute Dortmund and Full Professor at TU Dortmund
Scientist at Roche in chemical proteomics | Interested in drug discovery and interdisciplinary research | Oxford PhD
News and views from the Chemical Biology STP at the Francis Crick Institute
Synthetic organic chemistry at the University of Groningen and the Stratingh Institute for Chemistry | #ChemNobel 2016 | Making smart molecules & forging stereocenters since '88
https://benferinga.com
dad, professor, chemistry, biology
Chemical biologist at Tufts University working on small molecules, peptides, and biotechnology.
https://orcid.org/0000-0003-2878-6781
https://chem.tufts.edu/kritzer-lab