Excited to share this highlight of a beautiful study into how myeloperoxidase acts as a chromatin transformer
20.11.2025 14:45 — 👍 7 🔁 3 💬 0 📌 0
Thanks Weijie!
18.09.2025 15:43 — 👍 0 🔁 0 💬 0 📌 0
Besides the really cool science, this was a fun collaboration within the @maxplanck.de between @mpiib-berlin.mpg.de and @mpi-dortmund.bsky.social.
And I guess it is only the beginning, as there are still many mysteries of NETs that @garth-burn.bsky.social, Sebastian and I want to uncover...
18.09.2025 15:38 — 👍 4 🔁 0 💬 0 📌 0

Immunofluorescence images of CF patient sputum samples stained for DNA and MPO, respectively. The layover on the right shows that MPO and DNA nicely overlap.
As a final note, this whole process is highly relevant not only for killing pathogens, but also in autoimmune diseases.
We could also show that sputum of cystic fibrosis patients contains high levels of MPO functionalised NET-like structures.
18.09.2025 15:38 — 👍 2 🔁 2 💬 1 📌 0
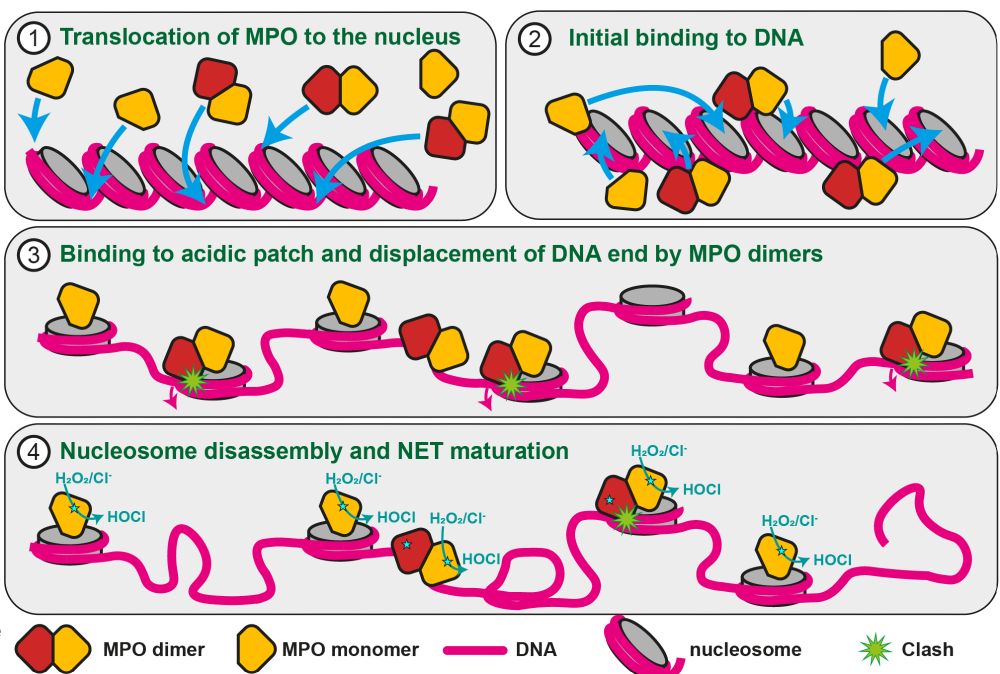
Scheme of the NETosis mechanism and MPO's dual role in it. Panel (1) shows chromatin and MPO monomers and dimers binding to it. In panel (2) we see that both will bind to the acidic patch on nucleosomes. Step (3) is the decondensation by prevention of hitone stacking upon MPO binding. In the case of MPO dimers, there is a clash with the nucleosomall DNA. (4) nucleosomes bound by MPO dimers have mostly disassembled, so that almost only MPO monomers are still attached to mature NETs. Their role is the production of reactive oxygen species at the site of inflammation.
MPO monomers, a minor fraction of the native MPO pool, can also bind to nucleosomes.
But, unlike dimers, they cannot disassemble the nucleosomes and remain stably associated.
This means that most nucleosomes will be evicted during NETosis, but some are protected by MPO monomers and end up in NETs
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0
Taken together, our data explain how MPO can achieve its dual role in NETosis (i.e. the generation of NETs) and be also a constitutent of mature NETs. The key lies in the dimerization!
MPO dimers, which are most abundant in neutrophils, will destabilized the nucleosome's DNA and lead to disassembly
18.09.2025 15:38 — 👍 0 🔁 0 💬 1 📌 0

On the left, we see a segmented tomogram of a NET. The tomogram is very crowded. In the center we have a massivebundle of protein filaments. It is surrounded by a tight meshwork of DNA filaments that that are decorated with protein particles. In addition, the tomogram contains also vesicles and granules.
On the right side, a cryo-ET reconstruction of a nucleosome is show featuring a prominent extra density that would be in agreement woth bound MPO. However, the resolution of the reconstruction does not allow to be certain.
Finally, we went back into "real" NETs and saw by #CryoET that (1) NETs are highly complex and contain more than just decondensed chromatin, and (2) that they contain nucleosomes decorated with something that might be MPO.
18.09.2025 15:38 — 👍 1 🔁 1 💬 1 📌 0

DNA deprotection assay in which a nucleosome was assembled with a DNA carrying a restriction site. Only when the DNA is unwrapped, it can be cleaved by the restriction enzyme. In the picture, we see a DNA gel in which we have lanes with one band (uncut long nucleosomeal DNA9 or two lower bands (cut DNA pieces). The left two lanes are uncut and cut DNA as marker. In the third lane, the nucleosome has been expose to MPO dimers, and the band pattern shows that all DNA is cut, meaning the DNA was unwrapped from the nucleosome. In the next five lanes, different concentrations of monomeric MPO were used, but in none the DNA was strongly cut, meaning that monomeric MPO cannot unrwap it from the nucleosome. The right two lanes are negative controls using catalase and HRP, respectively.
We see this disorder of the DNA terminus in all our reconstructions when MPO dimers are present, and only then. A strong indication that this is what actually destabilises the nucleosome!
Indeed, only MPO dimers and not monomers can lead to unwrapping of DNA, making it accessible for nucleases:
18.09.2025 15:38 — 👍 1 🔁 1 💬 1 📌 0
A tiny difference in the nucleosome structures is that once the second MPO unit is present, there would be a clash with the DNA terminus.
As a consequence, in the presence of MPO dimer the DNA has to locally unwrap and becomes disordered, as visible in this morph:
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0

Structure of the native MPO dimer bound to nucleosome
MPO dimers bind to the nucleosome in an identical way as the monomers we had seen before.
But the second MPO unit contacts the DNA at several positions. And this is important for understanding how it can destabilise the nucleosome.
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0
After changing experimental conditions, we could determine intermediate structures of native MPO with nucleosomes. And we found several molecular species in these samples:
1. Free nucleosomes and MPO
2. MPO monomers bound to nucleosome
3. MPO dimers bound to nucleosome
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0

Electron micrograph from cryo-EM of nucleosomes incubated with native MPO. Almost no nucleosomes are visible, but a lot of free DNA and small proteins representing MPO and probably also histones. On the right, representative 2D classes of MPO dimers are shown with a filamentous thin density next to them, which is DNA.
Okay, great. But what about native MPO? Does it behave similarly as the recombinant one?
Not exactly! Instead, it rapidly disassembles nucleosomes when we reconstitute both, leaving behind only MPO bound to DNA! That was a surprise, since we did not add any other factors such as ATP.
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0
We then reconstituted nucleosomes with a recombinant MPO that resembles a monomeric precursor of native (dimeric) MPO.
By #CryoEM, we saw that MPO stably binds to the acidic patch of the nucleosome, an a binding mode similar to many other nucleosome interactors.
18.09.2025 15:38 — 👍 1 🔁 1 💬 1 📌 0

Micrographs of super-resolution fluorescence microscopy (STED). Three panels represent stainig for MPO (left) and nucleosomes (middle) as well as a layover of both (right). The nucleosome channel revelas a fine meshwork representing NET filaments. MPO correlated with these filaments, but stains only discrete spots.
The first thing we found is that MPO associates with nucleosomes in mature NETs.
This interaction is direct and requires intact nucleosomes.
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0

Cartoon view of myeloperoxidase (MPO). MPO is a homodimer, each monomer consists of a heavy and a light chain. It contains a heme cofactor.
Intriguingly, #myeloperoxidase (MPO) is a key player both for the chromatin decondensation during NETosis and it also decorates mature NETs.
It produces reactive oxygen species and is one of the most important bactericidal enzymes of neutrophils.
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0
So, in essence, neutrophils transform their own chromatin into a weapon against pathogens.
But how exactly does this work? This is where we came into play.
Together with our colleagues (and now good friends) from @mpiib-berlin.mpg.de, we uncovered important steps in NETosis.
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0

Scheme of how NETosis works. Neutrophils contain many granules that are filled with bactericidal enzymes. Once the cells are stimulated by the presence of pathogens, these granules release their content to the extracellular space, but also into the nucleus.
Inside the nucleus, MPO and other factors lead to decondensation of chromatin, i.e. eviction of nucleosomes.
The nucleus disintegrates and subsequently, the neutrophil bursts open and releases the decondensed chromatin to the extracellular space. There the NET can entrap the pathogen. As NETs are functionalized with bactericidal enzymes, they can directly kill pathogens they contact.
During #NETosis, neutrophils decondense their #chromatin by disassembling #nucleosomes. Then, their nucleus breaks down and the cells burst open. The decondensed chomatin then forms the NET.
As #NETs are sticky and functionalised with bactericidal enzymes, they can trap and kill bacteria.
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0

Schematic drawing of a neutrophil cell and its mechanism of action. Neutrophils can leave the bloodstream to reach sites of infection/inflammation. They can uptake bacteria by phygocytosis and digest them afterwards. Also, they contain granules filles with bactericidal enzymes. Once those are released to the extracellualr space, they generate reactive oxygen species that are cytotoxic. They also release cytokines to further stimulate the immune response. And finally, they can decondense their chromatin in a process calles NETosis and release this decondensed chromatin to the extracellular space as so-called Neutrophil extracellular traps (NETs) that can entangle and kill bacteria.
Neutrophils are the master killers of the innate immune response. They can hunt down bacteria and eliminate them using a wide range of mechanisms, including phagocytosis and the release of ROS-generating enzymes including.
And they can form so-called Neutrophil Extracellular Traps (NETs).
18.09.2025 15:38 — 👍 0 🔁 1 💬 1 📌 0

Myeloperoxidase transforms chromatin into neutrophil extracellular traps - Nature
Myeloperoxidase, a highly expressed neutrophil protein, disassembles nucleosomes, facilitating neutrophil extracellular trap (NET) formation, and binds stably to NETs extracellularly.
We just published this paper of which I am immensely proud: www.nature.com/articles/s41...
In the study, we uncover how the enzyme MPO disassembles #nucleosomes in a process called NETosis, a funky mechanism of #neutrophils to kill #pathogens that you might have never heard of.
Let's dive in... 🧵
18.09.2025 15:38 — 👍 16 🔁 4 💬 1 📌 0

PostDoc Position (m/f/d) in Structural Biology of Membrane Protein Complexes
Main focus of the job will be Cryo-EM of membrane protein comolexes as well as protein/nucleic acid complexes involved in innate immunity.
For more information, check this:
www.mpi-dortmund.mpg.de/news/jobs/po...
01.07.2025 19:47 — 👍 3 🔁 3 💬 0 📌 0
I have an open postdoc position in my group at @mpi-dortmund.bsky.social @maxplanck.de!
If you are passionate about structural biology and/or membrane proteins, apply today!
The position is initially funded for two years, starting from this October. Deadline for application is on July 15th.
01.07.2025 19:47 — 👍 6 🔁 6 💬 1 📌 0
Reminds me of something that happened a long, long time ago in Tübingen 🙈
21.11.2024 22:37 — 👍 1 🔁 0 💬 1 📌 0
Yes, indeed. Thank you!
04.11.2023 17:23 — 👍 0 🔁 0 💬 0 📌 0
Thanks Eugene :)
04.11.2023 11:48 — 👍 1 🔁 0 💬 0 📌 0
3/3 This is work done by my truely outstanding trainee Milena during her Master's project. I consider myself very lucky that she will stay on for her PhD. Also, I am very grateful to Stefan for allowing me do to further develop as a scientist in his department!
04.11.2023 10:58 — 👍 1 🔁 0 💬 1 📌 0

Without γ1, Slo1 is activated in excitable cells synergistically by depolarization and intracellular Ca2+ binding. In the resting state, a charge gradient over the membrane exists with negative net charges on the intracellular side and positive charges on the extracellular side. This favors the resting state of the voltage-sensor domains of Slo1 in which the three arginine gating charges reside close to the intracellular side of the membrane. Depolarization pulls the gating charges towards the extracellular side and rearranges the entire VSD. This is synergistically enhanced by binding of Ca2+ to the gating ring.
In non-excitable cells with a constant resting state transmembrane potential, the polybasic stretch in γ1 locally changes the membrane potential and induce a similar movement of the gating charges towards the extracellular side as during action potential. Thus, the need for depolarization is decreased or completely absent and only Ca2+ binding is necessary to open Slo1.
2/3 The most striking feature of the structure highly positively charged polybasic stretch on the intracellular side of the membrane.
This stretch could locally change the voltage potential across the membrane and allow Slo1 to open without membrane depolarization!
04.11.2023 10:57 — 👍 0 🔁 0 💬 1 📌 0
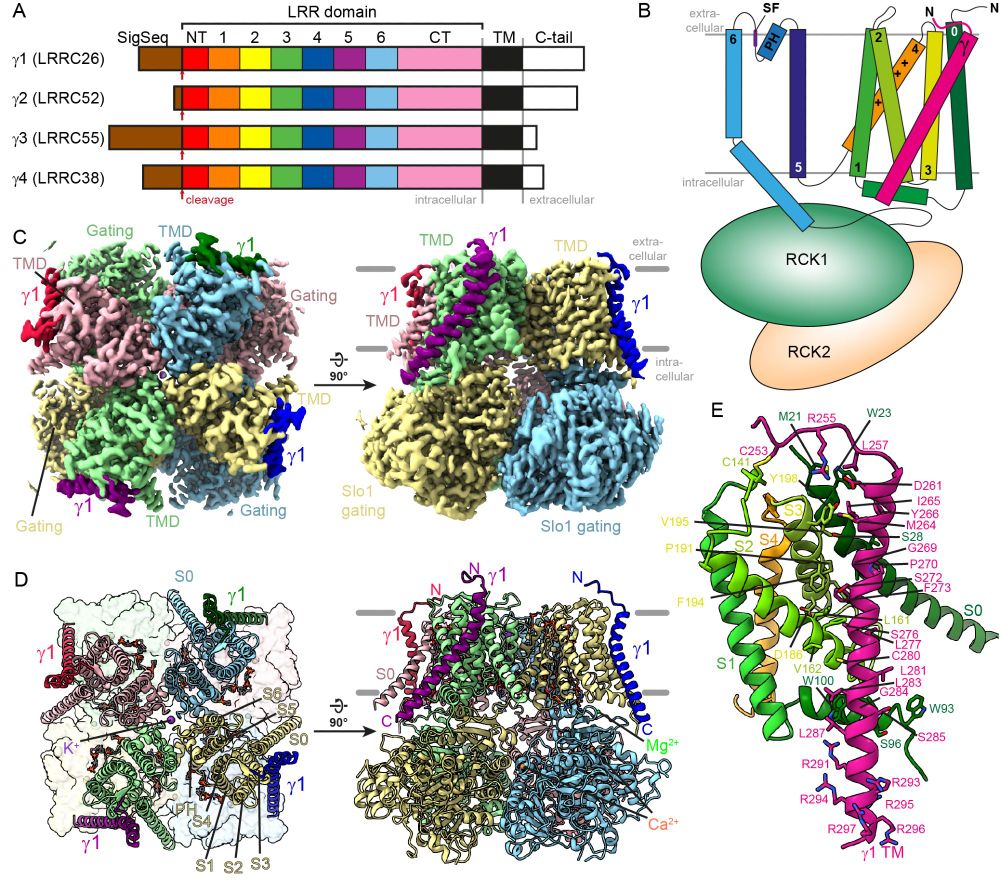
Figure 1 of the linked preprint showing an overview of the structure of the Slo1-γ1 complex.
A) Schematic representation of γ subunit domain organization
B) Cartoon scheme of γ1 helix binding across the Slo1 voltage sensor
C) EM density showing γ1 transmembrane helices binding to Slo1
D) Same depicting the molecular model as cartoon
E) Closeup of one γ1 transmembrane segment including transmembrane helix, extracellular hook and intracellular polybasic stretch binding to a Slo1 voltage-seonsor
1/3 Today I can proudly present the first preprint from my project group!
biorxiv.org/content/10.1...
We determined the CryoEM structure of the Slo1 potassium channel in complex with the regulatory subunit γ1 and propose a mechanism of how γ1 can activate Slo1.
04.11.2023 10:56 — 👍 17 🔁 3 💬 2 📌 0

Figure 1 from the linked paper. It shows the main targets of neurotoxic insecticides, which are mainly ion channels belonging either to the pentameric ligand-gated ion channel family or the tetrameric voltage-gated ion channel family.
Together with Stefan Raunser, I wrote a little review on neurotoxic insecticides acting on ion channels.
We focus on structures and mechanisms and try to reveal similarities in modes of action between chemically diverse compounds. In the end, many act surprisingly similar!
rdcu.be/doHyK
16.10.2023 16:43 — 👍 4 🔁 3 💬 0 📌 0
Biochemistry. Structural Biology in all its possible flavours. University of Göttingen. University Medical Centre Göttingen. Previously @MPI Dortmund and @MPI _Biochem.
Bioinformatics, protein modeling, cryoEM, drug screening, function prediction. Daisuke Kihara, professor of Biol/CS, Purdue U. https://kiharalab.org/ YouTube: http://alturl.com/gxvah
Scientist at the Max Delbruck Center for Molecular Medicine @mdc-berlin.bsky.social in Berlin.
Professor in Charité – Universitätsmedizin Berlin
Biophysics, data and structural biology at its coolest
Group Leader @MRC_LMB /Structural Biologist/Biochemist. Amateur baker in free time #RNAworld #spliceosome #telomerase #telomeres #cryoEM #Xraycrystallography
milkshake, boys, yards, schreiben über Patriarchat (erst der Dinge, jetzt der Mythen)
1/2 von @femshelfcontrol.bsky.social
Foto: Andrew Collberg
Assistant Professor at McGill University/ Goodman Cancer Institute studying myeloid cells in breast and ovarian cancers. 🇬🇧 in 🇨🇦
Postdoc in Raisch lab MPI Dortmund, cryoEM/cryoET, ion channels
New PI @IMB in Mainz, Germany. Combining in vitro and in vivo approaches to study maternal mRNA regulation in 🐟 embryos.
EMBO is the organization of more than 2,100 leading researchers that promotes excellence in life sciences in Europe and beyond.
https://www.embo.org/
Postdoc @mpiib in Berlin, graduated from Lu lab @UT Austin, and Deng Lab @Peking University. Focus on neutrophil innate immunity, DNA/RNA biofunctions
Group leader of Roderer lab @FMP-berlin.de, Germany
Interests: Life Science, structural biology, cryo-EM, membrane (glyco)proteins, endocytosis, host-pathogen interaction. Views are my own.
🐭 Original hessische Liebmaus | Schriftstellerin & Biologin 🪲| 💚 Podcast: @bugtales.fm | Kolumnen: hortarium.de & schreibersnaturarium.de | Hier gibt’s Hunde, Kunst, Garten, Natur, Wissenschaft & Bücher 📚 | Passionate Gamer 🎮 | https://jasmin-schreiber.de
PhD student @bjmurphylab.bsky.social @mpibp.bsky.social with @imprs-cbp.bsky.social
Interested in #cryoEM 🔬❄️, redox and membrane proteins
Cryo-EM @Prof Daniel Wilson Lab, Uni of Hamburg, Germany
Single particle cryo-EM, cryo-electron tomography
The life and times of Steve the otter...
The official Bluesky account of the World Bollard Association™️. MERCH STORE - https://worldbollardassociation.team-togs.com/shop/storeorderform.php


















