Now in its final version @jacs.acspublications.org ! pubs.acs.org/doi/10.1021/...
03.11.2025 17:38 — 👍 8 🔁 2 💬 0 📌 0Florian Ruepp
@fruepp.bsky.social
Doctoral Student in Chemistry | Living for the mountains, cats & sunny days ⛰️🐈☀️
@fruepp.bsky.social
Doctoral Student in Chemistry | Living for the mountains, cats & sunny days ⛰️🐈☀️
Now in its final version @jacs.acspublications.org ! pubs.acs.org/doi/10.1021/...
03.11.2025 17:38 — 👍 8 🔁 2 💬 0 📌 0Thanks for highlighting our work!
08.09.2025 09:28 — 👍 0 🔁 0 💬 0 📌 0
#RobSelects preprint of the week #ChemRxiv: Comprehensive mechanistic study of the oxidative aminative cleavage of alkenes with a hypervalent iodine oxidant and ammonium carbamate. #orgchem https://doi.org/10.26434/chemrxiv-2025-6cdgb
07.09.2025 08:13 — 👍 1 🔁 1 💬 1 📌 0
Congrats to @fruepp.bsky.social, Vasily Grebennikov, Mykola Avramenko, Marc-Olivier Ebert "Kinetic, Spectroscopic, and Computational Investigation of Oxidative Aminative Alkene Cleavage Reveals an N-Iodonium-Iminoiodinane Pathway" now @chemrxiv.org - chemrxiv.org/engage/chemr...
02.09.2025 19:18 — 👍 15 🔁 6 💬 1 📌 1
IRMPD spectroscopy, TIMS, and DFT reveal tautomerization in protonated BOX ligands, refining our understanding of non-covalent interactions relevant to catalysis. @bdebreaker.bsky.social @vmgorbachev.bsky.social
doi.org/10.1002/hlca...

Congrats to @mixalhsmpogdos.bsky.social, @sevenroediger.bsky.social, @fruepp.bsky.social, Patrick, Nathalie, Fabio on this epic study of the factors governing reductive elimination - using causal inference, organometallics, electrochemistry, kinetics and DFT
@chemrxiv.bsky.social go.shr.lc/4j8O5MM
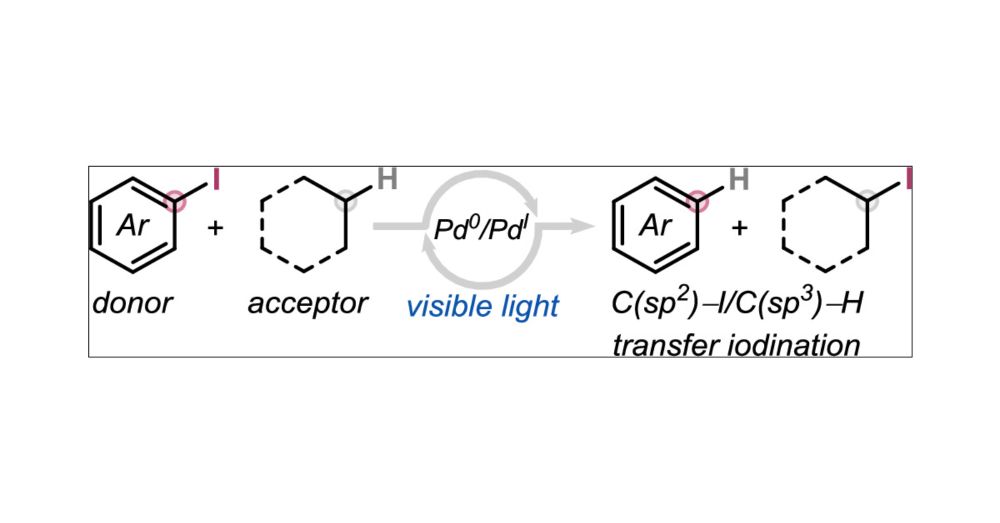
Many congrats to Emilien Le Saux for this new publication in @jacs.acspublications.org describing a catalytic approach to Transfer Iodination from Aryl Iodides to Nonactivated C(sp3)–H Bonds.
pubs.acs.org/doi/10.1021/...