Now published! www.nature.com/articles/s41...
Very nice collaboration with Hoogenboom ( @ucl.ac.uk ) and Bonev ( @uniofnottingham.bsky.social ) labs.
29.09.2025 14:24 — 👍 43 🔁 24 💬 1 📌 0

Description
Please note that job descriptions are not exhaustive, and you may be asked to take on additional duties that align with the key responsibilities ment...
I am looking for a motivated #Postdoctoral #Researcher to join my group at Imperial @imperialcollegeldn.bsky.social @imperiallifesci.bsky.social. The project focuses on #Antimicrobial #Materials & #Biomimetic #Interfaces
Please repost!
@imperialsci.bsky.social
www.imperial.ac.uk/jobs/search-...
19.09.2025 10:09 — 👍 5 🔁 9 💬 1 📌 1
If you are UK-based and working on any aspect of microbiomes (human, plant, insect, soil, animal, ...), please do sign up to Microbiome-Net for details of networking, funding and training opportunities.
forms.office.com/pages/respon...
13.09.2025 06:31 — 👍 34 🔁 39 💬 4 📌 0
Very pleased to see this out in @cmijournal.bsky.social - what non-antimicrobial options are there for recurrent #UTI, and what might be the role for #FMT? www.clinicalmicrobiologyandinfection.com/article/S119... @rohmaghani.bsky.social @imperialmdr.bsky.social @imperialbrc.bsky.social #MicroSky
29.07.2025 22:21 — 👍 5 🔁 2 💬 0 📌 0
Congrats! That's such great news! 😊
29.07.2025 15:31 — 👍 0 🔁 0 💬 1 📌 0
Yes, that's a good question! The elderly are potentially exposed to more antibiotics and could also have differences in their diet compared to younger adults, which could impact the availability of nutrients VRE are exposed to in the gut.
25.07.2025 09:18 — 👍 1 🔁 0 💬 0 📌 0
Great to see this work from Shiv Radhakrishnan and colleagues out in #GutMicrobes - using network analysis to explore #microbiome #metabolome interactions in an #IBD inception cohort: doi.org/10.1080/1949... @imperialmdr.bsky.social @imperialhepatology.bsky.social #IBDSky #GISky
18.07.2025 12:11 — 👍 2 🔁 2 💬 0 📌 0
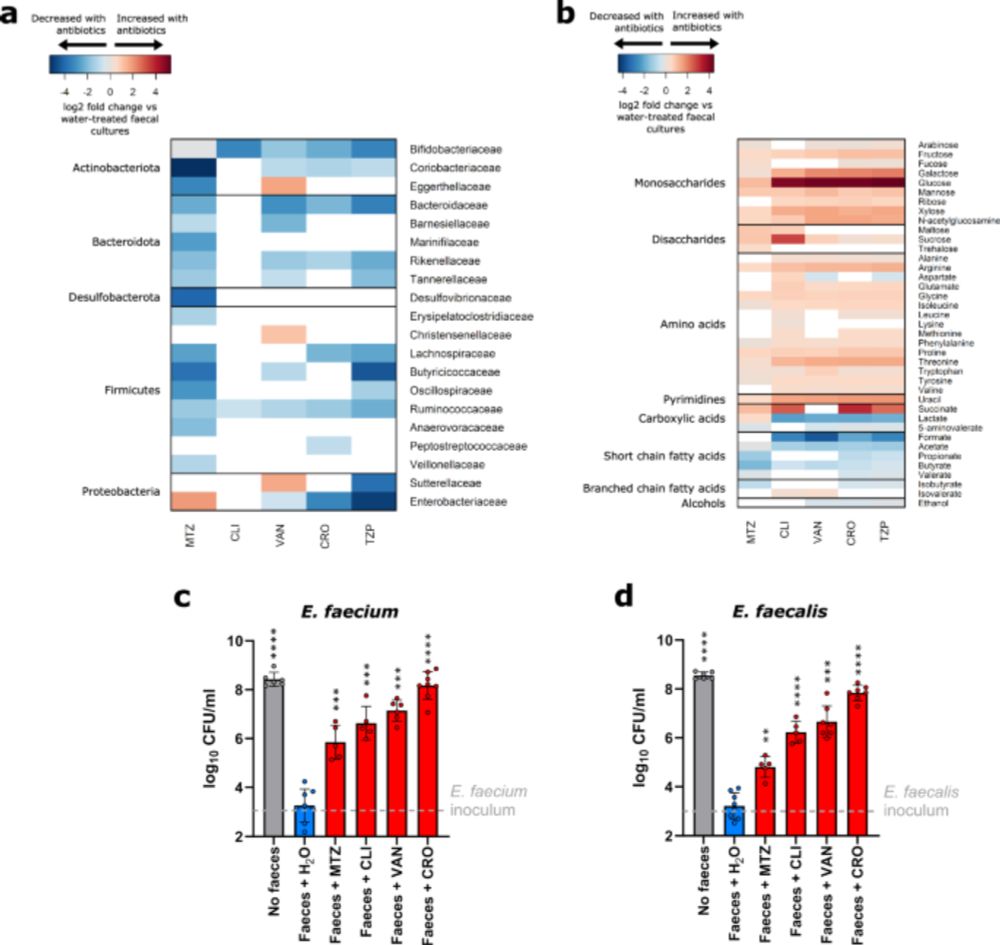
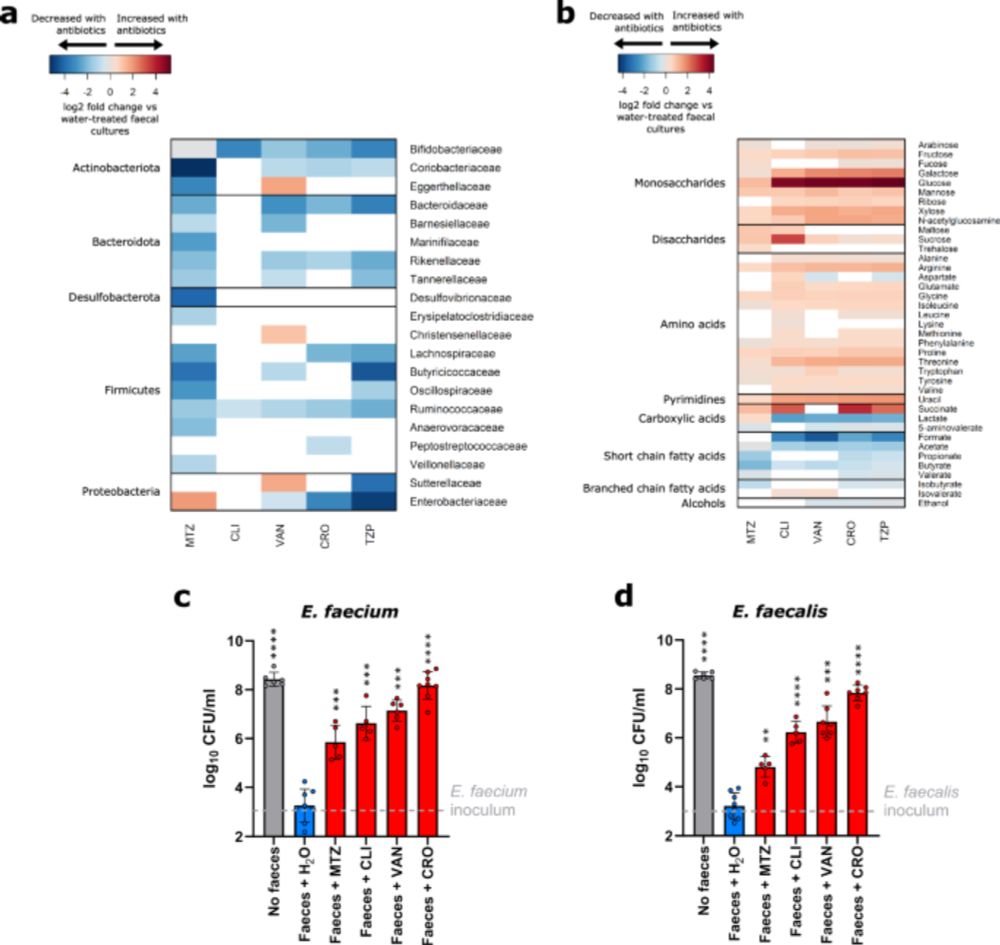
V nice study this. VRE growth in gut microbiome relies on different short chain fatty acids, which are themselves modulated by antibiotic exposure.
Complex but important to understand how antibiotic resistant bacteria persist in microbiome
@natcomms.nature.com 🧪 #AMR #ClinMicro #IDSky #UTISky
11.07.2025 05:38 — 👍 18 🔁 5 💬 0 📌 0
Vancomycin-resistant enterococci utilise antibiotic-enriched nutrients for intestinal colonisation - Nature Communications
Here, the authors show that vancomycin-resistant enterococci grow in the antibiotic-treated gut microbiome by utilising enriched nutrients in the presence of reduced concentrations of inhibitory micro...
Vancomycin-resistant 𝙀𝙣𝙩𝙚𝙧𝙤𝙘𝙤𝙘𝙘𝙪𝙨 𝙛𝙖𝙚𝙘𝙞𝙪𝙢 (VRE) thrives in the antibiotic-perturbed gut
VRE gobbles up enriched sugars and amino acids, and loss of short-chain fatty acids (acetate, propionate, butyrate) eliminates natural growth brakes
Therapeutic angle: Prebiotic SCFA mixtures block VRE growth!
11.07.2025 13:08 — 👍 27 🔁 9 💬 1 📌 0
A great privilege to work with @julieakmcdonald.bsky.social lab on this new paper, now out in @natureportfolio.nature.com Communications - how #antibiotic-related changes in gut #nutrients and #metabolites promote #VRE colonisation: doi.org/10.1038/s414... @imperialmdr.bsky.social
10.07.2025 20:57 — 👍 7 🔁 3 💬 0 📌 1
This microbiome therapeutic could be composed of a mixture of gut commensals that can deplete nutrients (that were enriched with antibiotic treatment) and restore the production of inhibitory microbial metabolites (that were depleted with antibiotic treatment).
14.07.2025 09:11 — 👍 0 🔁 0 💬 0 📌 0
This study will help guide the rational design of new microbiome therapeutics that could be used to restrict VRE intestinal growth, and subsequently reduce the development of invasive VRE infections.
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
Finally, we showed that VRE occupied overlapping but distinct nutrient-defined intestinal niches, where VRE had high growth when cultured with other VRE species and when cultured with other multidrug-resistant pathogens (carbapenem-resistant Enterobacteriaceae).
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
Killing of gut commensals with antibiotics also resulted in reduced nutrient competition, which led to an increase in the concentration of a wide range of nutrients. We showed that VRE used most of these nutrients as carbon or nitrogen sources to support their growth.
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
We showed significant but incomplete suppression of VRE growth by individual metabolites that were decreased with antibiotic treatment. However, mixtures of metabolites provided complete or near complete suppression of VRE growth.
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
Killing of gut commensals with antibiotics resulted in reduced production of many microbial metabolites normally produced by gut commensals.
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
We showed that VRE colonise the antibiotic-treated intestine due to killing of gut commensals (members of the gut microbiome).
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
Broad-spectrum antibiotics significantly promote VRE intestinal colonisation, causing the intestine to act as a reservoir for VRE that seed difficult-to-treat infections, such as bloodstream infections.
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
We showed that intestinal niches occupied by VRE were defined by the abilities of VRE to use specific nutrients that were enriched with antibiotic treatment and their abilities to grow with reduced concentrations of inhibitory microbial metabolites.
14.07.2025 09:11 — 👍 0 🔁 0 💬 1 📌 0
Today, Professor Donal Wall, @uofglasgow.bsky.social attended a pitch session as part of the Science, Innovation and Technology Committee's 'Under the Microscope' inquiry to explain why they should be interested in microbiomes.
01.07.2025 14:16 — 👍 12 🔁 6 💬 1 📌 1
Newly published study by Prof Lindsay Hall @halllab.bsky.social and Prof Willem van Schaik @wvschaik.bsky.social finding that antibiotic resistant Staphylococcus haemolyticus bacteria are common in premature babies
www.birmingham.ac.uk/news/2025/wi...
30.06.2025 14:51 — 👍 13 🔁 7 💬 0 📌 0
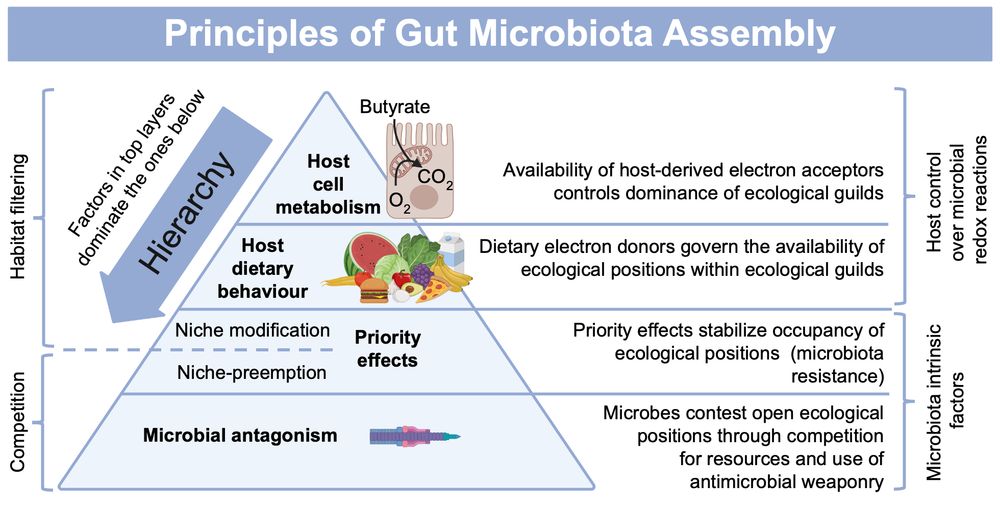
🔬🦠 How to make sense of the taxonomic complexity & interpersonal variability of the gut microbiota? We propose that beneath this complexity lies a hierarchy of factors that control gut microbiota assembly. Read more! #Microbiome #GutHealth
authors.elsevier.com/a/1km32_,2Ci...
14.03.2025 17:40 — 👍 28 🔁 11 💬 0 📌 2
Microbiology PhD student in Tung Le’s lab at the John Innes Centre - interested in all things Streptomyces and plasmid related 🔬🧫 She/Her
BBSRC Fellow and Assistant Professor in Soft Matter and Life-like Systems. / Dept. Life Sciences, Imperial College London /📍London 🇬🇧 https://continilab.com
Interested in the physiological role of bacterial efflux pumps and host-pathogen interactions🏳️🌈 (he/him)
https://scholar.google.com/citations?user=nhIW1SUAAAAJ&hl=en
Molecular Microbiologist at the Doherty Institute, University of Melbourne. All things Staph, Listeria, Enterococci, Phages and Sport.
Interested in microbial ecology & evolution. Views are only my own. (he/him)
🎓: https://scholar.google.com/citations?hl=en&user=OBPpZq4AAAAJ&view_op=list_works&sortby=pubdate
👨💻: https://github.com/raufs
Microbe lover 🦠 | Microbiology PhD | Postdoc at University of Minnesota in the lab of Julia Willett | First-gen | she/her
Microbiologist and microbiome host, culture expert (of the microbial variety), shameless cat lady, proud Mum to 2 amazing women 💪🇨🇦🧫🐈
Host-pathogen interactions | CRISPR screens
Interested in persistence mechanisms of parasites and bacteria, previously in the Treeck lab @Crick and IGC, now in the Rohn lab @UCL
Infectious Disease Epidemiologist investigating #AMR ~ Based at The Kids Research Institute Australia.
Interest in Australian politics and global affairs.
Servant to two Lebanese ex-street cats
reposts ≠ endorsement
https://orcid.org/0000-0002-5295-8451
Professor of Infectious Disease Dynamics at @lshtm.bsky.social. Developing methods for making sense of outbreak data. Outside of work trying to make sense of the rest of the world.
Scottish Immunologist based in The School of Infection & Immunity at The University of Glasgow.
Honorary Fellow at The Institute for Regeneration and Repair at The University of Edinburgh.
Infectious Disease Epidemiologist at University of Cambridge
Director of NDM Centre for Global Health Research & NIHR Global Prof at Oxford Uni
T cells, vaccines, melioidosis, AMR & global lab strengthening
Honorary Consultant at OUH - ID/micro/travel medicine
Immunology lab at the University of Edinburgh - infection, co-infection, regulation, metabolism - with a love of wild spaces too
http://www.peronalab.uk/
Physician Scientist | Professor of Infectious Diseases University of Sheffield | Principal Investigator MRC Unit The Gambia | Respiratory Viruses | Strep A | Vaccines and Immunity
https://www.sheffield.ac.uk/smph/people/clinical-medicine/thushan-de-silva
Professor of Clinical Pharmacology & Global Health. Maternal & Infant Lactation pharmaoKinetics (MILK). Equity. African women in science. Infectious diseases. Acute Medicine. Mother of 5. Christian.
Sir Henry Dale Fellow at #WCIP at the University of Glasgow. Interested in how Toxoplasma gondii uses iron.
Lab updates from the Walmsley Lab EdinUni_CIR & @edinuni-irr.bsky.social
Studying how oxygen and nutrient availability reprogram #neutrophils posts by @ajbrenes.com
Microbiome, drugs, phages, ecology, AI, the Bronx
Research Fellow at Northumbria University: phage, synthetic biology, sialic acid, transport proteins. He/him.