
Happy to announce our most recent preprint: Stereoselectivity and plasticity of a common binding pocket in TRPM3, by cryo-EM and elecrophysiology. Great collaboration with the Voets, Vriens and CISTIM labs.
www.biorxiv.org/content/10.1...
@janinebrunner.bsky.social
Group Leader VIB and Assistant Professor VUB Brussels, Belgium - Biochemist - Structural biologist - interested in #MembraneProteins #cryoEM - nature - paragliding

Happy to announce our most recent preprint: Stereoselectivity and plasticity of a common binding pocket in TRPM3, by cryo-EM and elecrophysiology. Great collaboration with the Voets, Vriens and CISTIM labs.
www.biorxiv.org/content/10.1...
Happy to share the latest from the lab, led by Daniel Alvarez, in collaboration with @lizconibear.bsky.social. In this AA-MD tour-de-force, we delve deep into the mechanism and energetics of lipid uptake by bridge-like lipid transfer proteins, and we learn a few interesting things along the way...
07.08.2025 08:45 — 👍 42 🔁 14 💬 1 📌 0We are excited to announce the first in-cell structure of a LINC complex within a native nuclear envelope at subnanometer resolution!! #In-cell cryo-ET + #atomistic MD simulations! @tomdendooven.bsky.social et al!!
www.biorxiv.org/content/10.1...
Happy to share the latest work from the lab, led by @mudgal17.bsky.social, in collaboration with the Weis lab @ethzurich.bsky.social.
How do nuclear membranes fuse during NPC assembly? We answer this question in our latest work, where we identify a new mechanism for membrane fusion… (1/13)

New preprint in the lab combining what we like best: insects, love, chemistry and the olfactory system! 1/8
www.biorxiv.org/content/10.1...
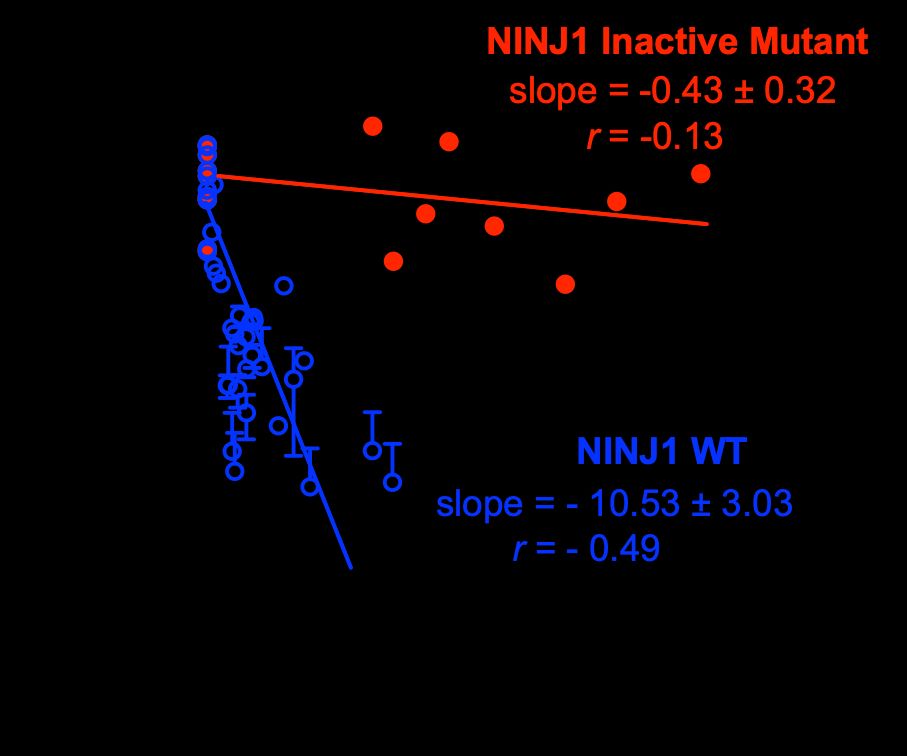

Excited to share our latest story in Nature. We developed a high-throughput cellular stretch system to look for genes regulating membrane rupture under tension, and found an amazing small protein called NINJ1 that weakens the membrane for rupture! H/T to Zozo, my 10-y.o. for her artist's impression😃
09.06.2025 15:19 — 👍 18 🔁 6 💬 2 📌 2Check out this remarkable debut paper from @jie-xu.bsky.social, a very creative lab alumnus. Very proud!
www.nature.com/articles/s41...
I’m thrilled to finally share this preprint! It contains a WOW Cryo-EM structure (likely one of the strongest protein fibers known to man 🤯), but we’ve also untangled its biological function: a novel type of virulence factor💀!
www.researchsquare.com/article/rs-6...
In our latest preprint we used cryoEM to solve the structure of A-ENA fibers and show that they are stabilized by 10 isopeptide bonds per monomer. A-ENA couples the spore to the cry-toxins, and in doing so increases the virulence of Bacillus thuringiensis: www.researchsquare.com/article/rs-6...
20.05.2025 09:22 — 👍 36 🔁 12 💬 1 📌 1Congrats to first author Stephan Schenck, and collaborators Toon Laeremans and @jansteyaert.bsky.social
05.05.2025 18:13 — 👍 0 🔁 0 💬 0 📌 0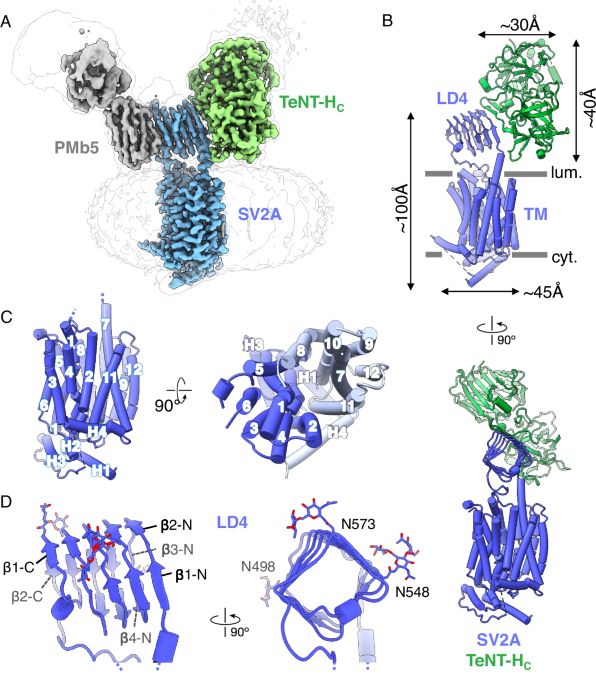
I am delighted to share our findings on the binding mode of tetanus neurotoxin and the anti-epileptic drug Levetiracetam to SV2A, an essential synaptic vesicle membrane protein of unknown function, now online in @NatComms.bsky.social
www.nature.com/articles/s41...
Please RT. Post-doc opportunity alert! 💥 Come join our team (thelowlab.org) at Imperial, London, working on bacterial secretion systems. The position is funded by the Wellcome Trust.
For more details and to apply please see
www.imperial.ac.uk/jobs/search-...
Here's our newest study: we reveal the molecular principles of neuronal excitation by glutamate, and how physiological temperatures influence this process.
www.nature.com/articles/s41...
www.nature.com/articles/d41...
Led by @anish-mondal07.bsky.social, published in @natureportfolio.nature.com
Structural basis of ClC-3 inhibition by TMEM9 and PI(3,5)P <sub>2</sub> pubmed.ncbi.nlm.nih.gov/40093093/ #cryoEM
18.03.2025 19:45 — 👍 1 🔁 1 💬 0 📌 0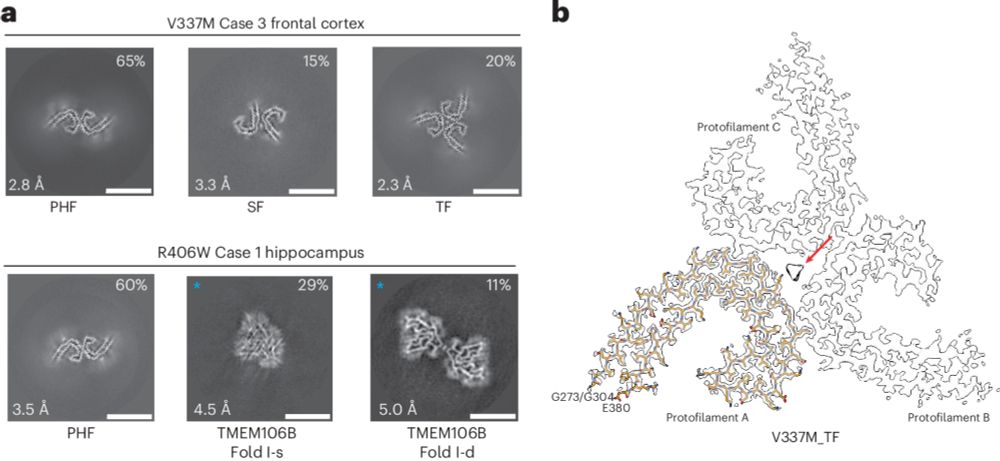
Happy to share our latest publication on
@naturesmb.bsky.social Natural Structure & Molecular Biology, we show tau filaments show Alzheimer fold in mutants V337M and R406W.
thanks @sjorsscheres.bsky.social and Michel Goedert's support. 🥳
rdcu.be/edqzW
rdcu.be/edoGv
Thrilled to share the structure of dimerised human PINK1 docked to an endogenous translocase array on the mitochondrial surface, composed of two TOM complexes, bridged by a VDAC2 dimer! Published today in Science www.science.org/doi/10.1126/...
@wehi-research.bsky.social @komanderlab.bsky.social
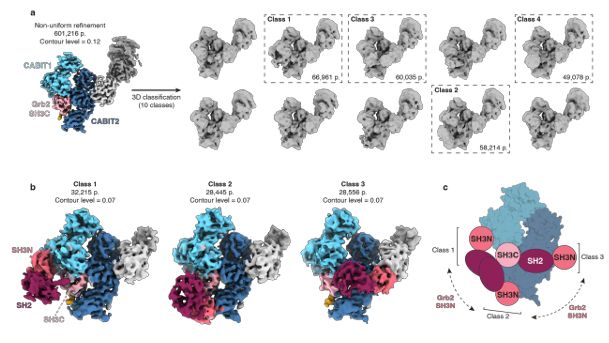
Themis and Grb2 form a constitutive structural hub in TCR signaling
doi.org/10.1101/2025...
@savvideslab.bsky.social
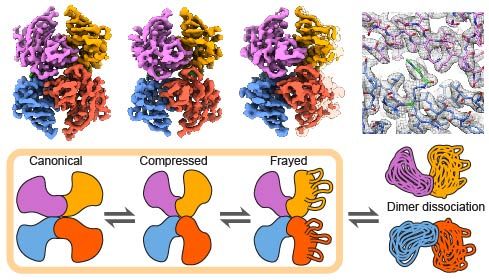
Just because it's a homo-oligomer, don't assume it's symmetric! @benjabsnt.bsky.social & collaborators used cryo-EM to study the transthyretin (TTR) tetramer (55 kDa), shedding light on its misfolding pathways linked to amyloid disease. Now in peer-reviewed form: www.nature.com/articles/s41...
22.01.2025 16:45 — 👍 62 🔁 17 💬 0 📌 0In a great collaboration with @hummerlab.bsky.social and the Kräusslich lab: HIV capsid doesn't break at the NPC; instead, it cracks open the NPC itself! Details in Cell: authors.elsevier.com/sd/article/S... @mpibp.bsky.social @uniheidelberg.bsky.social A thread below:
17.01.2025 18:43 — 👍 424 🔁 134 💬 7 📌 19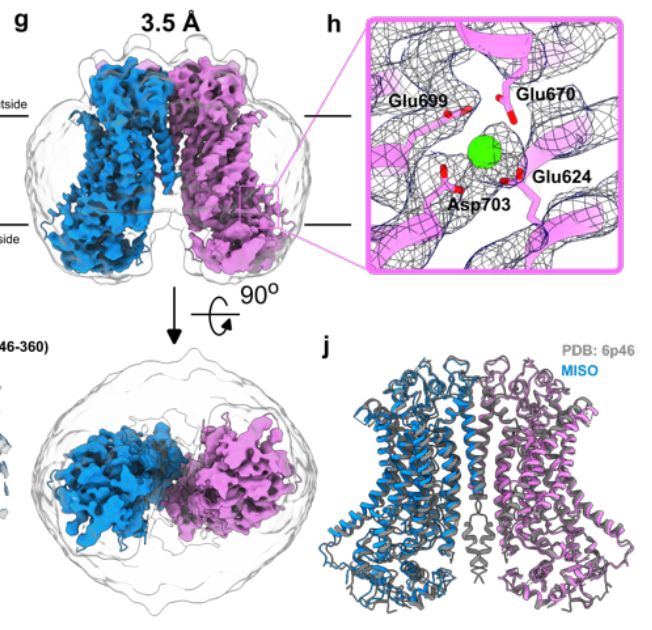
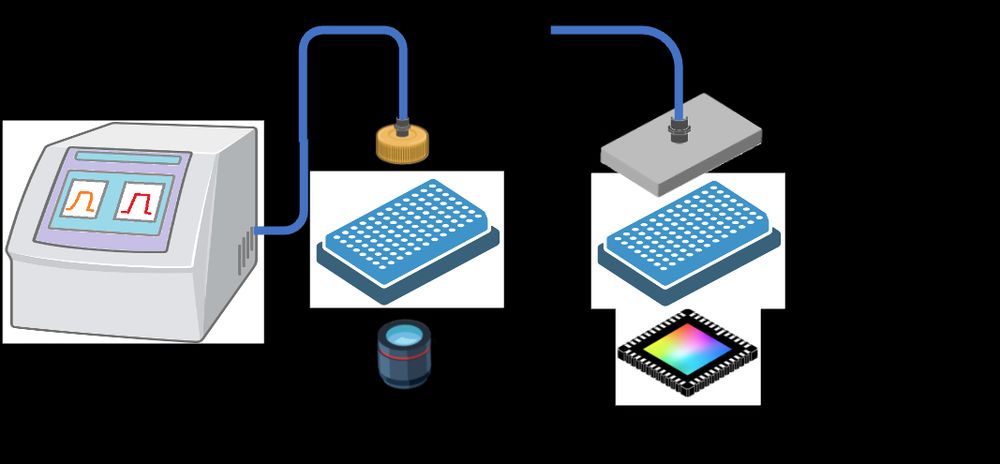
Protein production for cryoEM structure determination just got a lot cheaper - high resolution structure from half a plate of HEK cells with the "MIcro ISolation (MISO)" microfluidics-based approach. Congrats to the Brunner and Efremov labs in Brussels! www.biorxiv.org/content/10.1...
16.01.2025 22:57 — 👍 44 🔁 11 💬 0 📌 0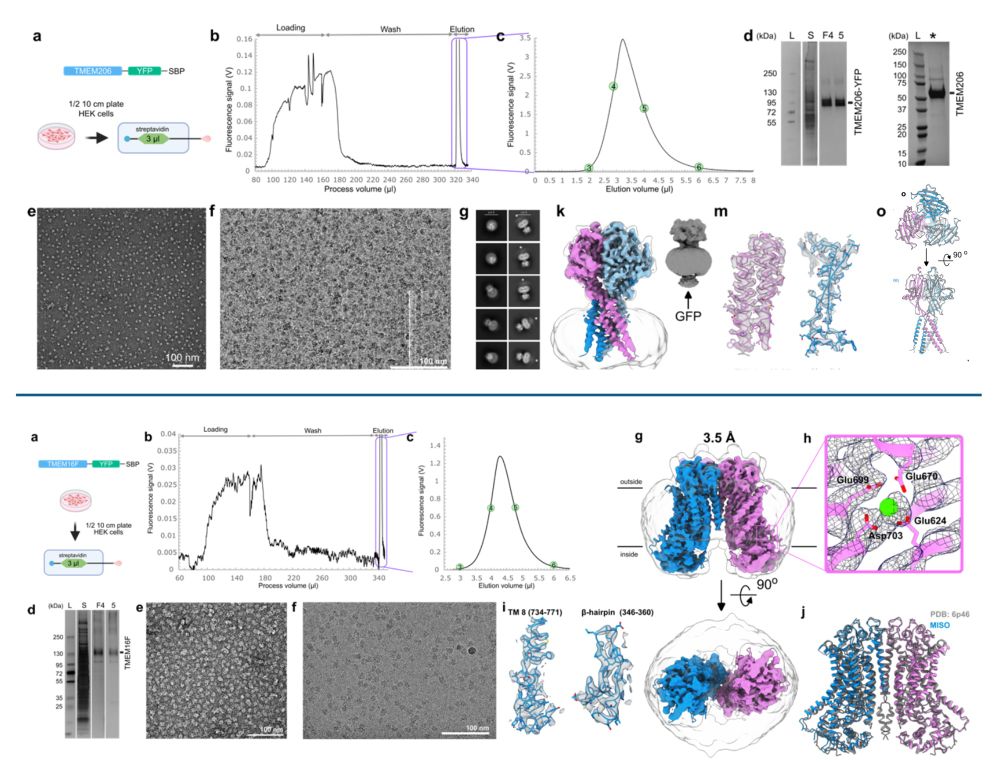
Cryo-EM structures of mammalian membrane proteins from a single cell culture dish?
Yes, Micro ISOlation (MISO) sample preparation makes it possible.
www.biorxiv.org/content/10.1...
Happy to be part of this exciting story and wonderful collaboration with the Rouslan Efremov lab.
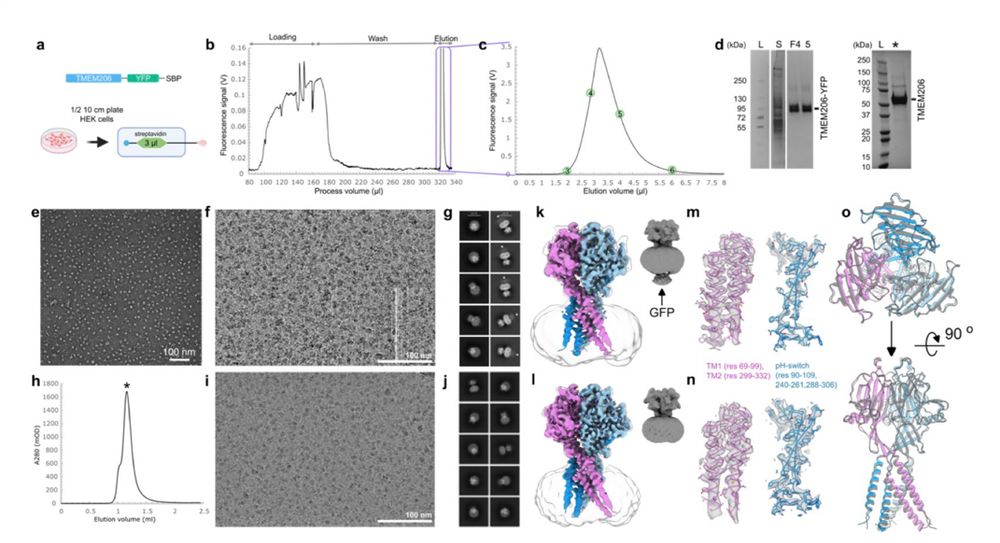
Love this.. 👏
MISO: Microfluidic protein isolation enables single particle cryo-EM structure determination from a single cell colony
www.biorxiv.org/content/10.1...