Proud to have been part of this work.
26.02.2026 15:32 — 👍 3 🔁 1 💬 0 📌 0Proud to have been part of this work.
26.02.2026 15:32 — 👍 3 🔁 1 💬 0 📌 0
Happy to announce that our latest paper is now out! Have you ever wondered how neural tissues control their size? In this paper, we show that cell division orientation is critical in both the cortex and retina. www.science.org/doi/10.1126/...
04.02.2026 19:16 — 👍 92 🔁 31 💬 5 📌 2Indeed.
30.01.2026 15:38 — 👍 13 🔁 0 💬 1 📌 0
Excited to finally share the final/final.doc version of our paper. It's been a journey, but very proud of the result. Well done to all involved, especially @elsieplace.bsky.social
@kchinnaiya.bsky.social , @thomasdwkim.bsky.social, @sethblackshaw.bsky.social 👏👏
www.nature.com/articles/s41...
Second paper is a collaboration with Jim Handa's lab at Hopkins. Cigarette smoke exposure induces aging-related molecular changes in both mouse and human retinal pigment epithelium.
www.pnas.org/doi/10.1073/...
I'd like to close by thanking lead author Nicole Pannullo, who recently defended her thesis, who with Debarpita Datta, @claytonsantiago.bsky.social, Jared Hangman, Lizhi Jiang, and Leighton Duncan -- and support from NIH -- made this possible./end
16.01.2026 21:06 — 👍 2 🔁 0 💬 0 📌 0
One reason why the Sox8/9 mutant phenotype is so much less dramatic than that of Nfia/b/x mutants may be persistent compensatory expression of other Sox family members, most notably Sox2, which might compensate for the loss of Sox8/9./11
16.01.2026 21:03 — 👍 2 🔁 0 💬 1 📌 0So what's going on here? While Sox8/9 suppress early-born cell type generation when misexpressed, and directly regulate transcription of NFI family genes, they are not required for maintaining late-stage temporal identity, being required for glial differentiation rather than specification./10
16.01.2026 20:58 — 👍 1 🔁 0 💬 1 📌 0
What about Sox8/9 function in mature Muller glia, where we previously showed that Nfia/b/x actively suppress neurogenic competence? As with Nfia/b/x mutants, we observe a modest induction of injury-induced proliferation, but no glial-derived neurogenesis or induction of Ascl1./9
16.01.2026 20:52 — 👍 1 🔁 0 💬 1 📌 0
scATAC-Seq and CUT&TAG data show that Sox8/9 predominantly function as transcriptional activators, in combination with other late-stage progenitor-specific factors such as NFIs. However, Sox8/9 mutants show no change in retinal cell fate specification or clear disruptions of temporal identity./8
16.01.2026 20:48 — 👍 1 🔁 0 💬 1 📌 0
Single-cell multiomic analysis of Sox8/9 double mutant glia show reduced Notch signaling and altered expression of many genes known to regulate cell adhesion and migration, as well as mature glial markers./7
16.01.2026 20:43 — 👍 1 🔁 0 💬 1 📌 0
Both Sox8 and Sox9 mutants resulted in progressive radial displacement of Muller glial cell nuclei towards the photoreceptor layer. This was enhanced in Sox8/9 double mutants./6
16.01.2026 20:38 — 👍 1 🔁 0 💬 1 📌 0Early retinal progenitor-specific loss of function of Sox9 did not affect neurogenesis or cell fate specification, and despite much effort, we were unable to generate early progenitor-specific Sox8 or Sox8/9 double mutants. We shifted instead to selectively deleting these genes in neonates./5
16.01.2026 20:36 — 👍 1 🔁 0 💬 1 📌 0
When over expressed in early-stage E14 progenitors, both Sox8 and Sox9 repress generation of early-born retinal ganglion and amacrine cells and promote formation of later-born photoreceptors. This matches what we previously observed with NFI factors./4
16.01.2026 20:30 — 👍 1 🔁 0 💬 1 📌 0
Overexpression, conditional loss of function, and biochemical analysis all confirmed that this was the case for Nfia/b/x in retina. We asked if this was also the case for Sox8/9./3
www.sciencedirect.com/science/arti...

Sox8/9 play an important role in regulating brain and spinal cord astrocyte and oligodendrocyte differentiation, often acting cooperatively with Nfia. Our multiomic analysis of retinal progenitors identified NFI and SoxE factor as more broadly promoting late-stage temporal identity, however./2
16.01.2026 20:23 — 👍 3 🔁 0 💬 1 📌 0
The lab's first paper of the new year is out. In it, we investigate the role of the late stage retinal progenitor-enriched SoxE family factors Sox8 and Sox9 in controlling retinal development./1
www.biorxiv.org/content/10.6...

We’re thrilled to share that CSHL Associate Professor Jeremy Borniger (@jborniger.bsky.social) has received the 2026 Bakewell Emerging Leader Award from The Mark Foundation. His lab is exploring synthetic torpor—a hibernation-like state—as a novel way to slow cancer growth. #ScienceMakesLifeBetter
13.01.2026 19:49 — 👍 8 🔁 3 💬 1 📌 0
Several stages of brain development in a chick embryo
New featured article!
Resolving forebrain developmental organisation by analysis of differential growth patterns
@emmanning.bsky.social @elsieplace.bsky.social @kchinnaiya.bsky.social @sethblackshaw.bsky.social @thomasdwkim.bsky.social
http://dlvr.it/TQLWh5
Congratulation!
08.01.2026 23:21 — 👍 0 🔁 0 💬 0 📌 0
Nice summary on our paper on mechanisms controlling development and evolution of the cone-dominant ground squirrel retina, which is now in final form at eLife.
www.lifescienceeditors.com/2026/01/06/h...
rdcu.be/eX8JD
DRN new publication alert:
Manning et al., 2025, @emmanning.bsky.social, co-led by Marysia Placzek and Elsie Place @elsieplace.bsky.social, in collaboration with Seth Blackshaw @sethblackshaw.bsky.social

A new paper from my lab. I will have a full description soon. rdcu.be/eX1Ld
07.01.2026 17:17 — 👍 42 🔁 14 💬 3 📌 1I want to give my deepest thanks to Tomomi Shimogori -- with whom this all started nearly 20 years ago -- and more recently Marysia and Elsie. It has been an honor and privilege to work with you all.
22.12.2025 17:23 — 👍 2 🔁 0 💬 0 📌 0I have no illusions that this will stop the ongoing trickle of prosomere-promoting single-color in situ hybridization studies in one Frontiers journal or another. I hope this will lead the Allen Brain Atlas to finally revise their developmental mouse reference atlas, however.
22.12.2025 17:21 — 👍 3 🔁 0 💬 1 📌 0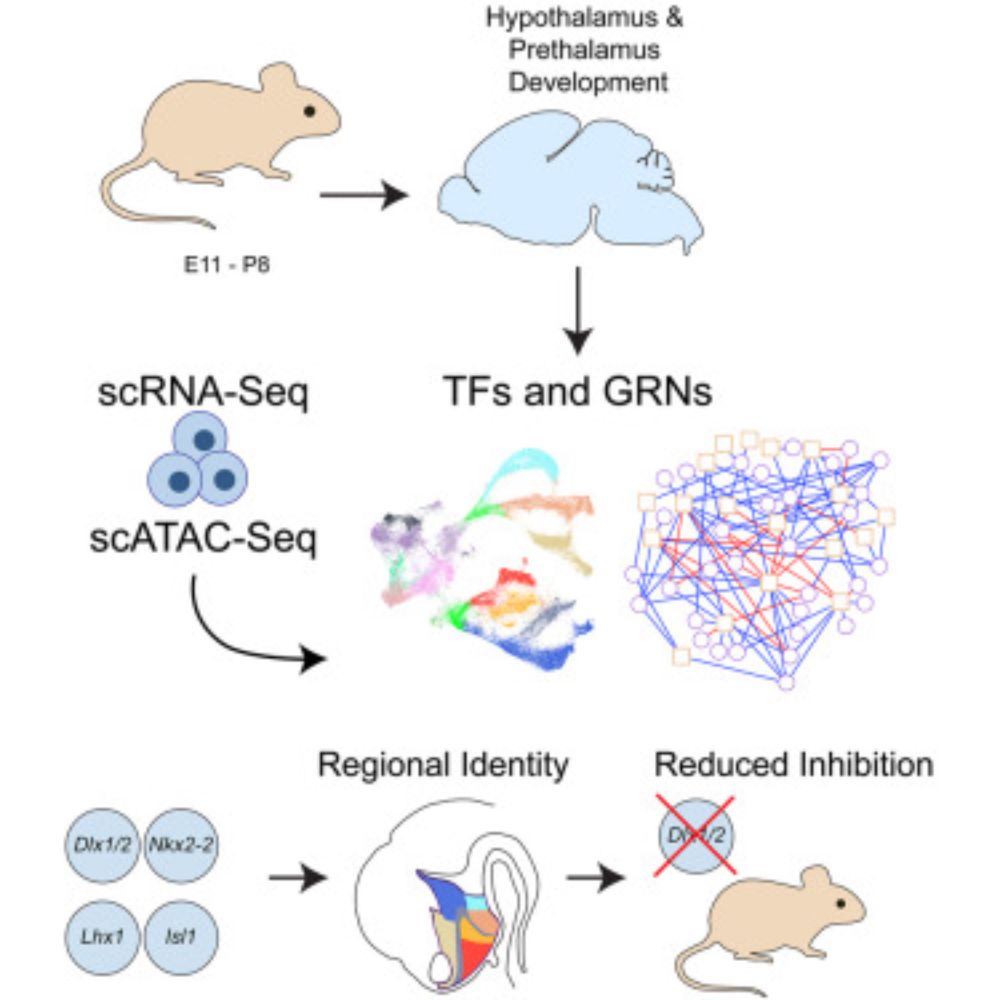
And finally combining multiomic-based gene regulatory network and genetic analysis in mouse earlier this year:
www.cell.com/cell-reports...

Moving to chick here:
www.cell.com/cell-reports...

Moving to single-cell analysis in mouse:
www.nature.com/articles/s41...

Starting here: www.nature.com/articles/nn....
22.12.2025 17:16 — 👍 3 🔁 0 💬 1 📌 0Contrast with the prosomere model, which puts that telencephalon and hypothalamus as dorsal and ventral halves of the "secondary prosencephalon". The data here match the conclusions drawn from what's now a long series of molecular studies of both mouse and chick hypothalamic development.
22.12.2025 17:15 — 👍 1 🔁 0 💬 1 📌 0