
a man in a suit and tie is giving a thumbs up sign .
ALT: a man in a suit and tie is giving a thumbs up sign .
I’d also like to thank all coauthors for their work on this manuscript, including lab alumni Drs. Jacob Landeck and Xingchen Liu, grad student Krishna Anand, and high school intern Sasha Litvak; UMass Chan cryo-EM facility staff Drs. Song, Chang & Ouch; and you for reading! (16/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 0 📌 0

a man wearing a hat is playing a clarinet in front of a saxophone .
ALT: a man wearing a hat is playing a clarinet in front of a saxophone .
I’d also like to point out that Josh never picked up a pipette before joining my lab! His PhD is in Mech Eng and purely computational! Yet in a short time he mastered biochem such that he was developing badass assays and his grid prep skillz are copied by others around UMass. (15/16)
07.07.2025 19:06 — 👍 1 🔁 0 💬 1 📌 1

a woman wearing a sweater that says thank you on it
ALT: a woman wearing a sweater that says thank you on it
At a time when science funding is so precious, we want to acknowledge that our work is NIH NIGMS funded, @joshua.pajak.bsky.social is funded by an American Cancer Society postdoc fellowship, and our MD sims used resources provided by NSF @accessforci.bsky.social and UMass Chan SCI. (14/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 1

RFC is conceptually analogous to a GTPase, which has dedicated Nucleotide Exchange Factors and NTPase Activating Partners. What makes RFC unique is that its own substrates are the NEF (PCNA) and NAP (DNA). (13/16)
07.07.2025 19:06 — 👍 1 🔁 0 💬 1 📌 0
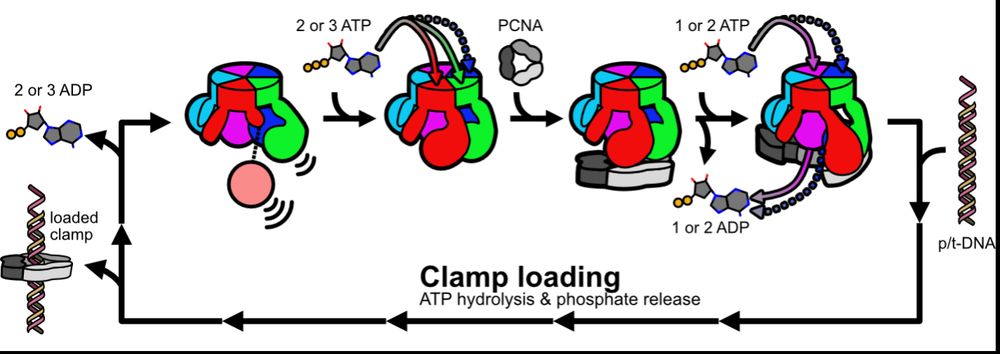
Based on our data, we propose a model whereby fast ATP binding to some subunits readies RFC to bind PCNA, and then PCNA completes RFC’s nucleotide exchange en route to clamp loading. We propose this prevents futile ATP hydrolysis and helps ensure RFC binds PCNA before DNA. (12/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
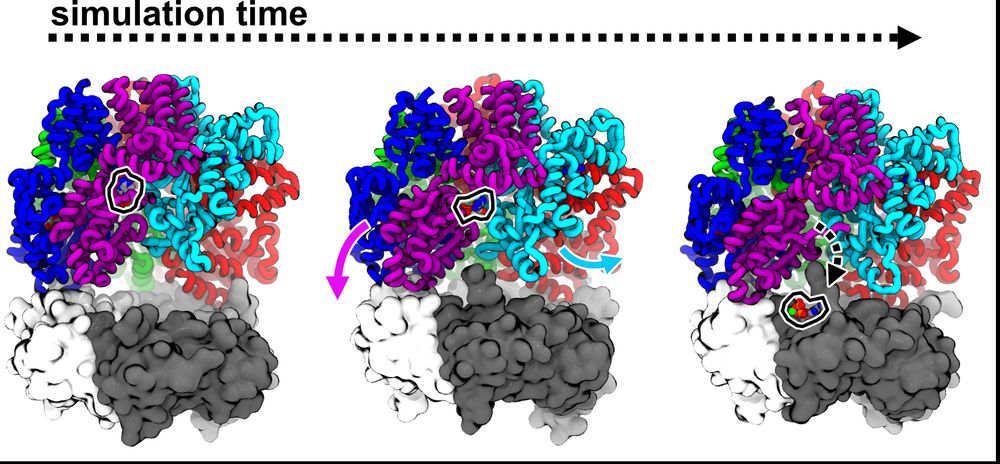
Josh performed more MD sims to try to understand how PCNA could promote nucleotide exchange in RFC. His sims predict that the D/E interface weakly bridged by ADP can be pried open as D binds PCNA, accelerating release. (11/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0

woody from toy story is standing in front of a dresser and says `` well , it 's about time ! ''
ALT: woody from toy story is standing in front of a dresser and says `` well , it 's about time ! ''
Thus, “PCNA is a Nucleotide Exchange Factor for Clamp Loader ATPase Complex”! It only took us 10 posts to get to the title of the paper😉. But how does PCNA do this? (10/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
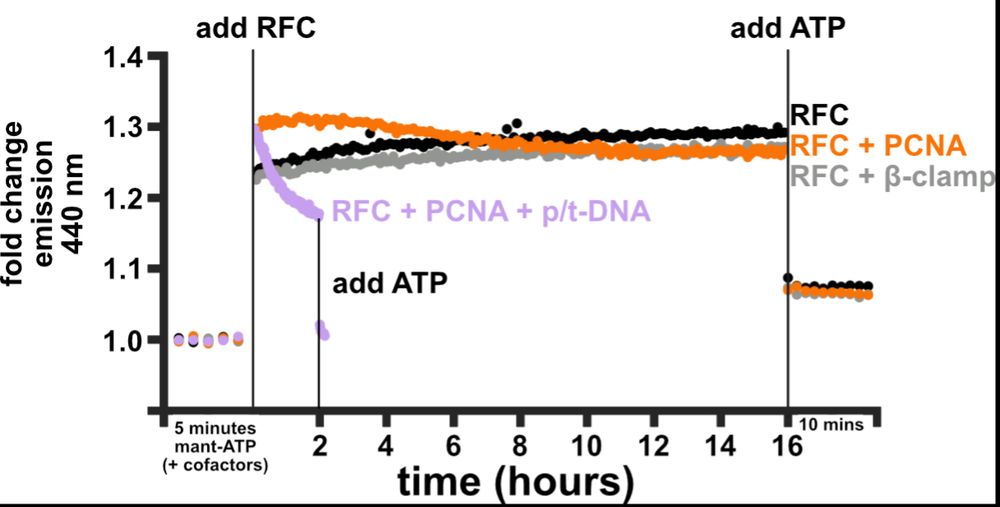
So, what catalyzes nucleotide exchange? Josh developed a FRET assay using a labeled ATP analog to monitor ATP binding to RFC. RFC alone binds some ATPs fast but then binds more ATP slowly, consistent with A&B (and maybe C) exchanging faster than D. PCNA speeds up these slow binding steps! (9/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
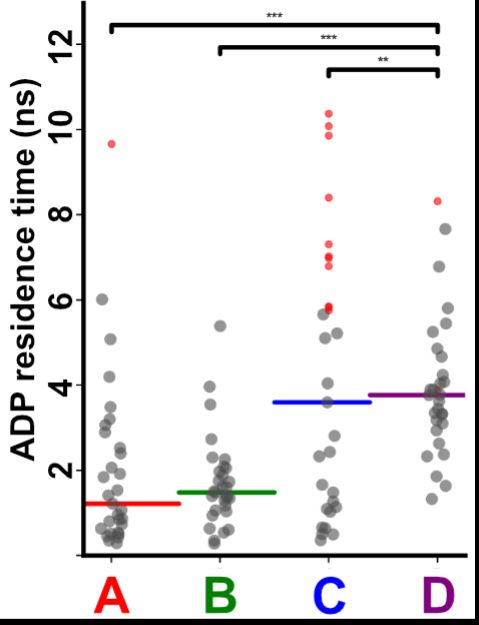
To test how interface opening plays a role in ADP release, Josh performed tRAMD(doi.org/10.1021/acs....), which is a technique that predicts relative release rates. He found that the half-off rate for C or D is slower than A or B, consistent with his cryo-EM. (8/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
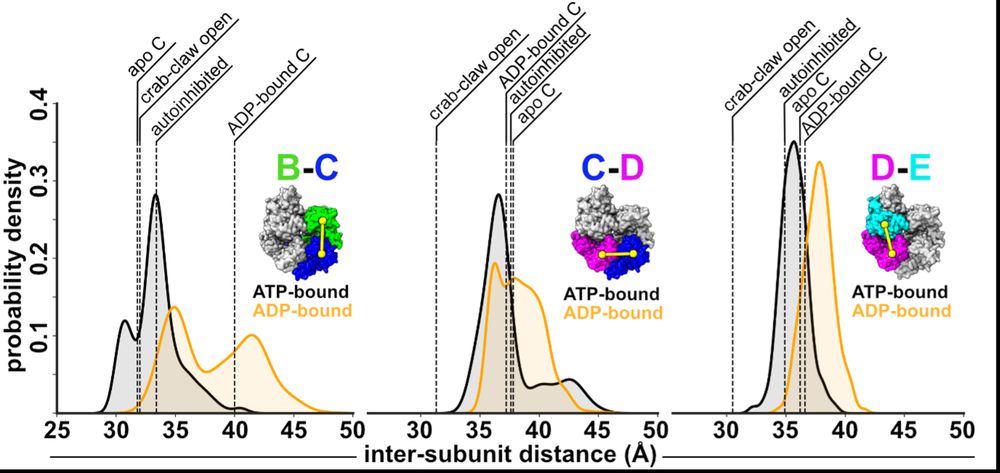
Next, we wondered how the dynamics of RFC influence ADP release? So, Josh performed MD sims. His sims predict that ATP-interfaces are tighter than ADP ones, and that the B/C interface can open farthest, offering an explanation why this interface is apo in our cryo-EM reconstructions. (7/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
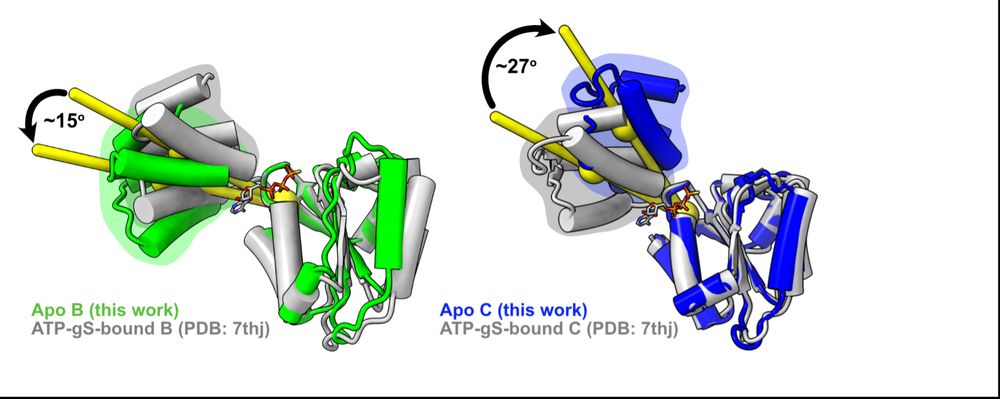
First, we looked at how RFC subunits respond to nucleotide binding by comparing Josh’s apo subunits to our lab’s previous ATPgS-bound subunits and found that the B&C subunits’ lids rotate in opposite directions! We had never seen this in a AAA+ before (if you know of any, please let us know!) (6/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0

scooby doo and his friends are looking for something in the dirt
ALT: scooby doo and his friends are looking for something in the dirt
We wanted to understand the functional relevance of why they hold ADP tightly, so we started looking for clues, perhaps the most fun part of science. (5/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
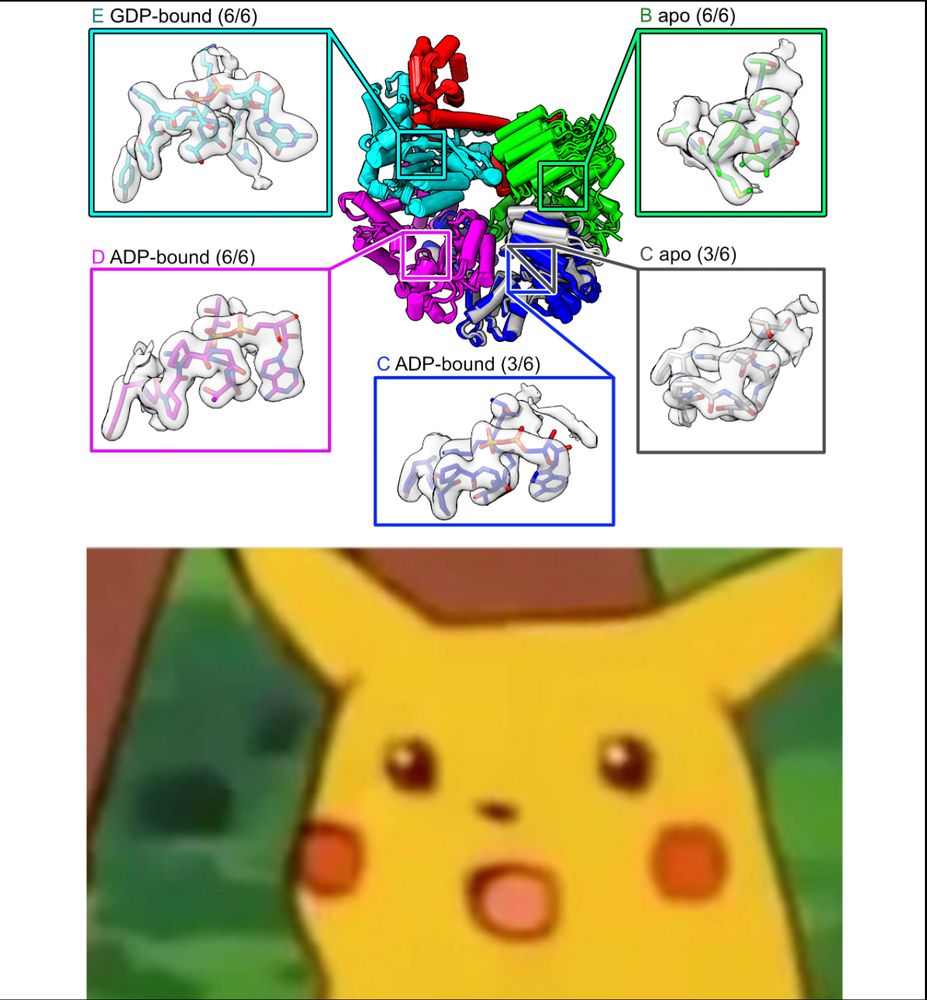
Imagine our surprise when we saw that the C and D subunits were ADP-bound! Because he never added nucleotide, these ADPs must have co-purified with RFC over a 3 day prep, meaning that RFC releases ADP very slowly from these subunits. (4/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
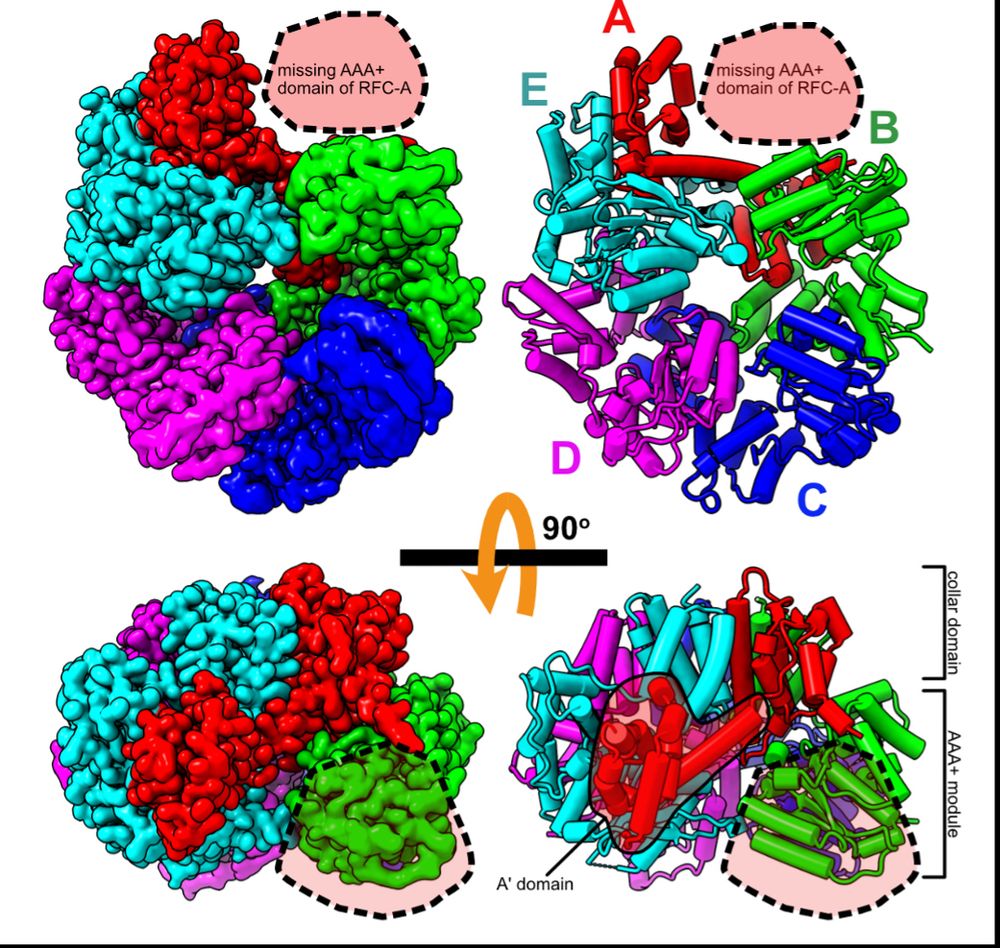
To get insights into RFC recycling, postdoc @joshuapajak.bsky.social determined the structure of RFC in the apo state. He got great overall resolution of the core RFC complex, but the AAA+ domain of the A subunit is missing, suggesting it is flexibly tethered when apo. (3/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0

Recently, our lab showed how an ATP-bound RFC loads PCNA onto DNA during replication and repair (doi.org/10.7554/eLif...). But RFC loads PCNA extremely fast, so that when RFC has done its job once, how does it prep for the next round? (2/16)
07.07.2025 19:06 — 👍 0 🔁 0 💬 1 📌 0
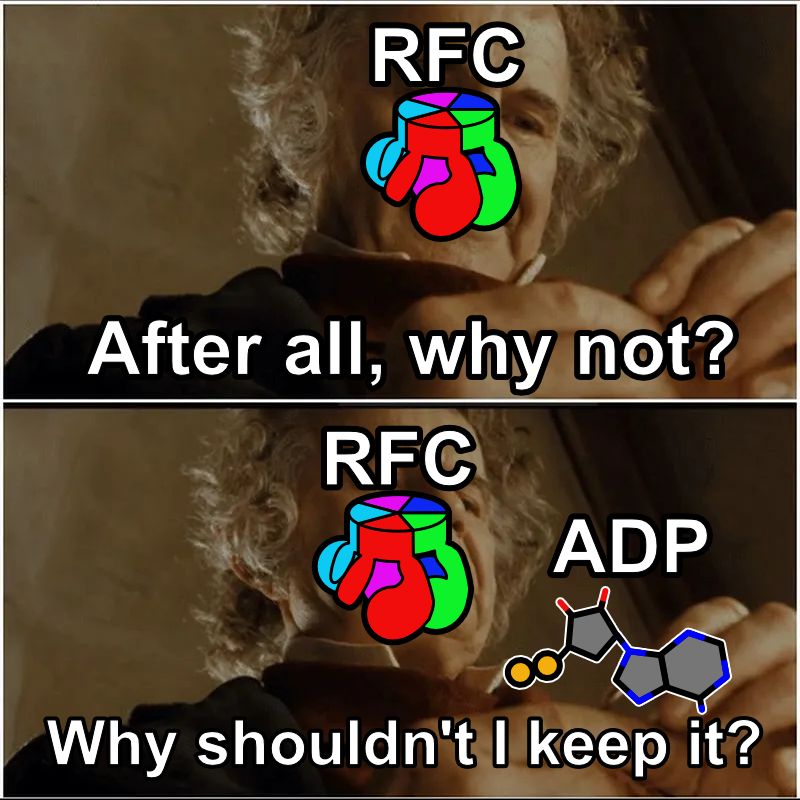
Every day we make enough DNA to go to the moon and back. So, all our DNA replication enzymes work fast, right? In our latest preprint, we show that a key enzyme, the clamp loader ATPase RFC, releases ADP much slower than it loads PCNA. What gives? (1/16)
doi.org/10.1101/2025...
07.07.2025 19:06 — 👍 8 🔁 0 💬 1 📌 1
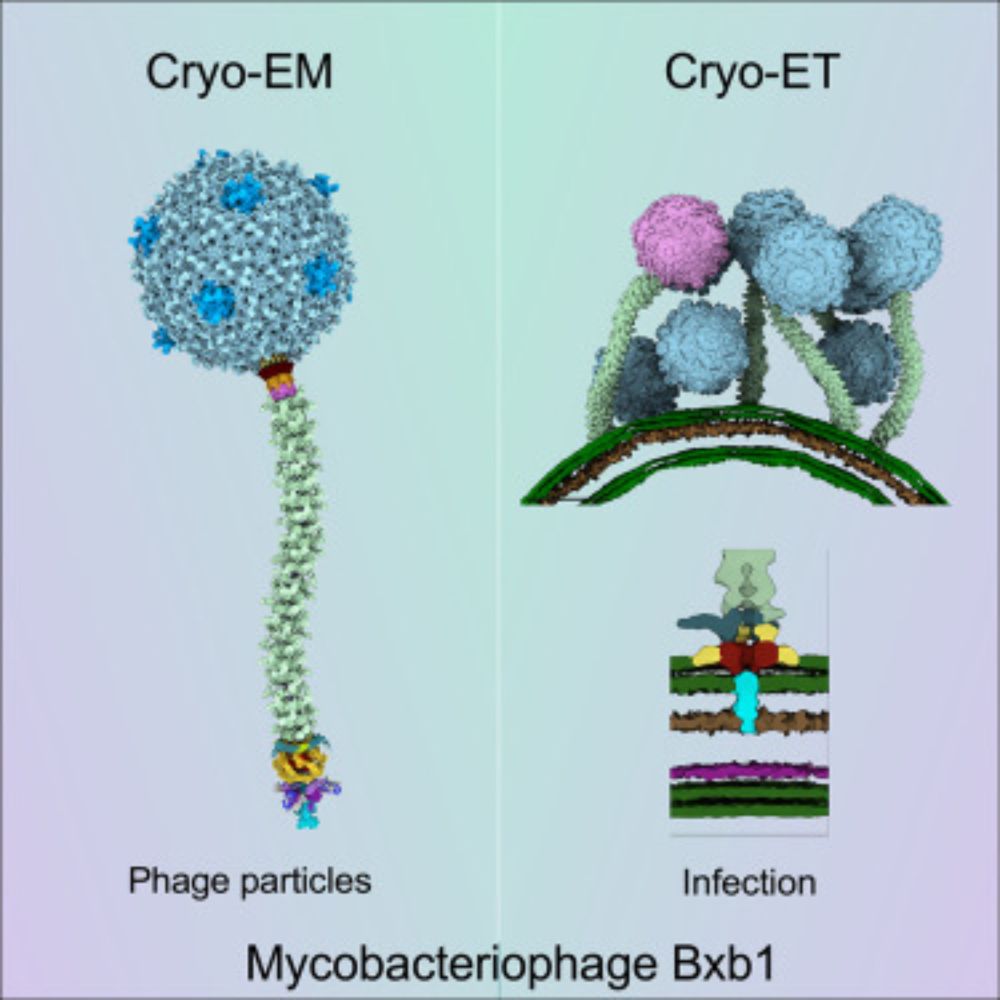
Structure and infection dynamics of mycobacteriophage Bxb1
Mycobacteriophage Bxb1 is a well-characterized virus of Mycobacterium smegmatis with double-stranded DNA and a long, flexible tail. Mycobacteriophages…
Hurray, it is finally out! Meet bacteriophage Bxb1 - the subject of my first full-phage cryo-EM study. My structures are beautifully complemented by the Park Lab’s cryo-ET analysis, shedding light on mycobacteriophage structural changes during infection.
www.sciencedirect.com/science/arti...
16.04.2025 12:50 — 👍 71 🔁 28 💬 5 📌 4

a cat is standing next to a bottle and says `` thank you !!! '' .
ALT: a cat is standing next to a bottle and says `` thank you !!! '' .
It’s been a long road, so massive props to everyone who worked on this project, including lab alum Emily Agnello, PhD, graduate students Julia Hobaugh and Rakeyah Ahsan, and Chen Xu, PhD and Kangkang Song, PhD at the fantastic UMass Chan CryoEM facility! /13
18.04.2025 14:24 — 👍 4 🔁 0 💬 1 📌 0
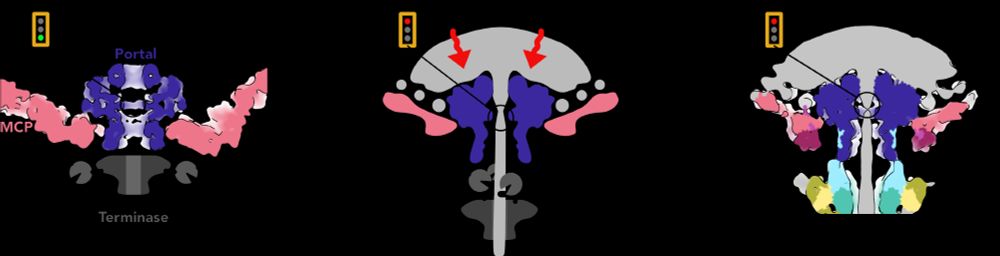
Putting that together with decades of research into phage genome packaging, we propose a speculative model for how the Portal switches from DNA-pumping mode to Tail attachment mode, using the pressure-induced piston motion of Portal to displace the motor. /12
18.04.2025 14:22 — 👍 3 🔁 0 💬 1 📌 0
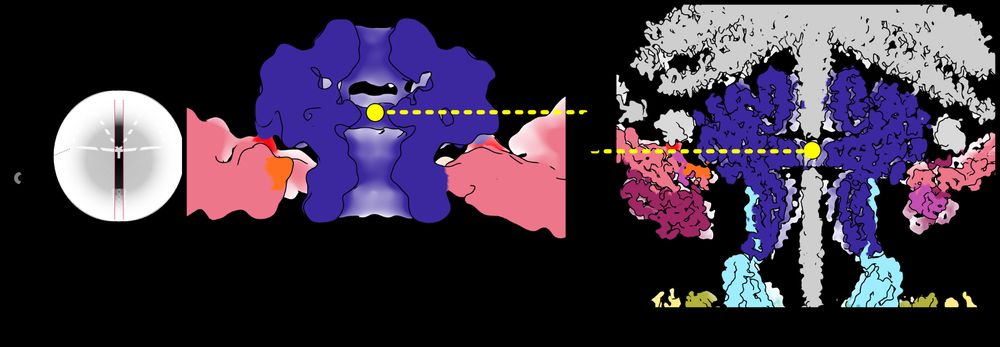
So how does the Portal know it’s time to close the pore? We got a clue by comparing the position of the Portal in capsids before and after DNA has been pumped in. The pressure from DNA pushes the Portal lower in the capsid aperture like a piston! /11
18.04.2025 14:20 — 👍 3 🔁 0 💬 1 📌 0
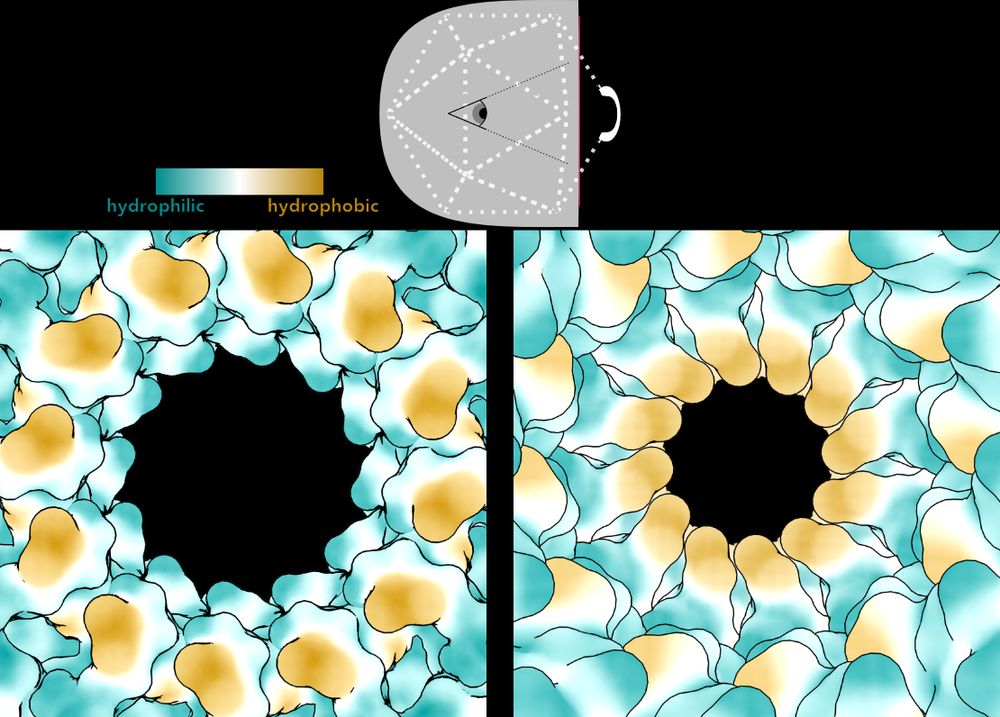
On the left, Bayfield et al. showed the pore through Portal in an early, immature capsid. A specialized motor pumps the genome into the capsid through this open, hydrophilic pore. Then, it constricts in the mature phage, becoming narrow and hydrophobic. /10
18.04.2025 14:19 — 👍 3 🔁 0 💬 1 📌 0
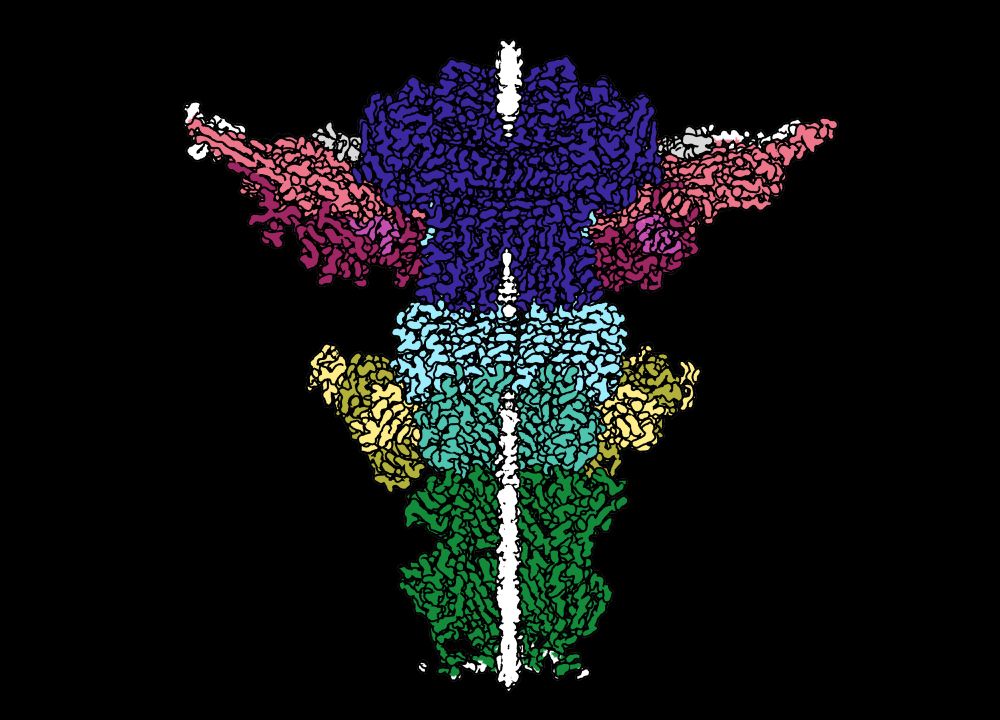
In 2020, Ollie Bayfield and Fred Antson predicted that one component of the Neck complex, the Portal protein (dark blue), would be constricted to hold DNA in the capsid of this phage. And they were right! /9
Bayfield et al elifesciences.org/articles/55517
18.04.2025 14:18 — 👍 3 🔁 0 💬 1 📌 0
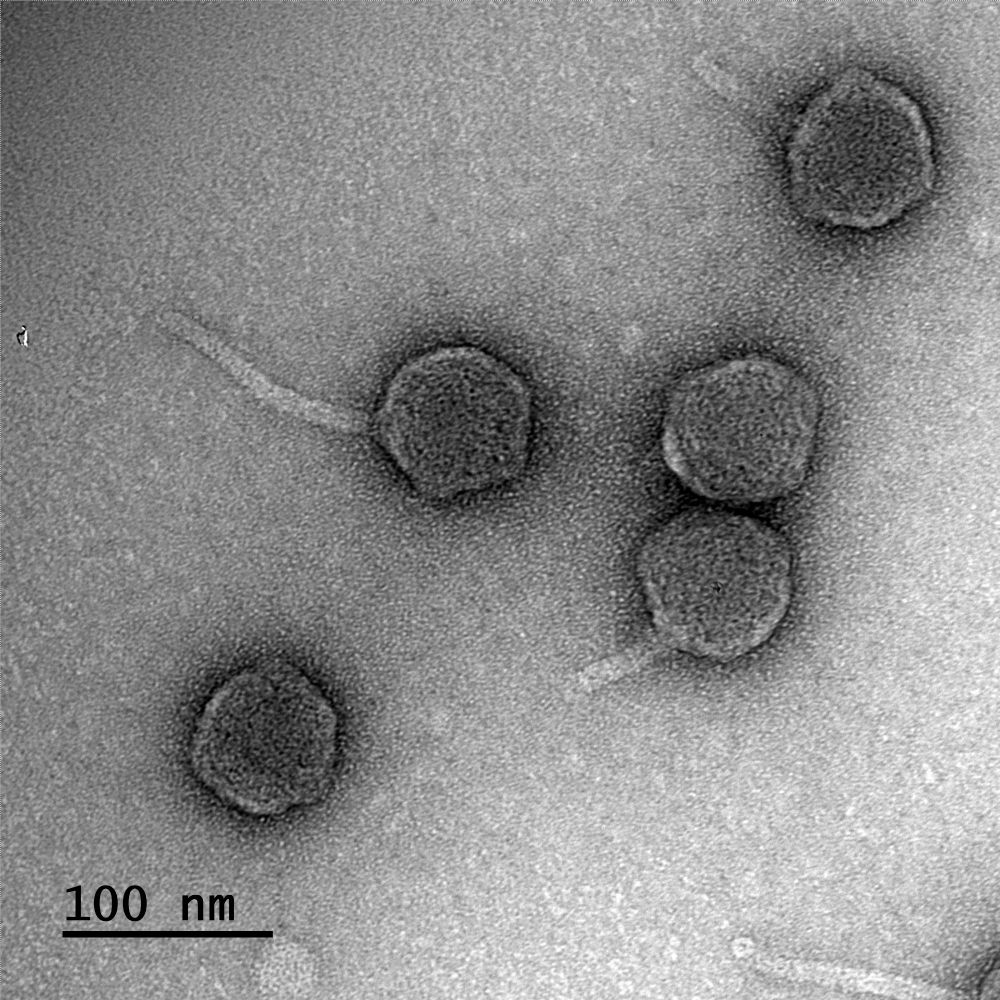
We know that the Neck of this phage is sufficient to hold its DNA inside the capsid because we also isolated phage that have broken tails, and therefore nothing left but the Neck to retain DNA. /8
18.04.2025 14:17 — 👍 5 🔁 0 💬 1 📌 0
But the phage has another problem—it needs a channel through the Neck and Tail to inject its genome into the host when it infects, but it can’t shoot its shot until it finds the right host. How does it keep that channel closed? /7
18.04.2025 14:16 — 👍 3 🔁 0 💬 1 📌 0
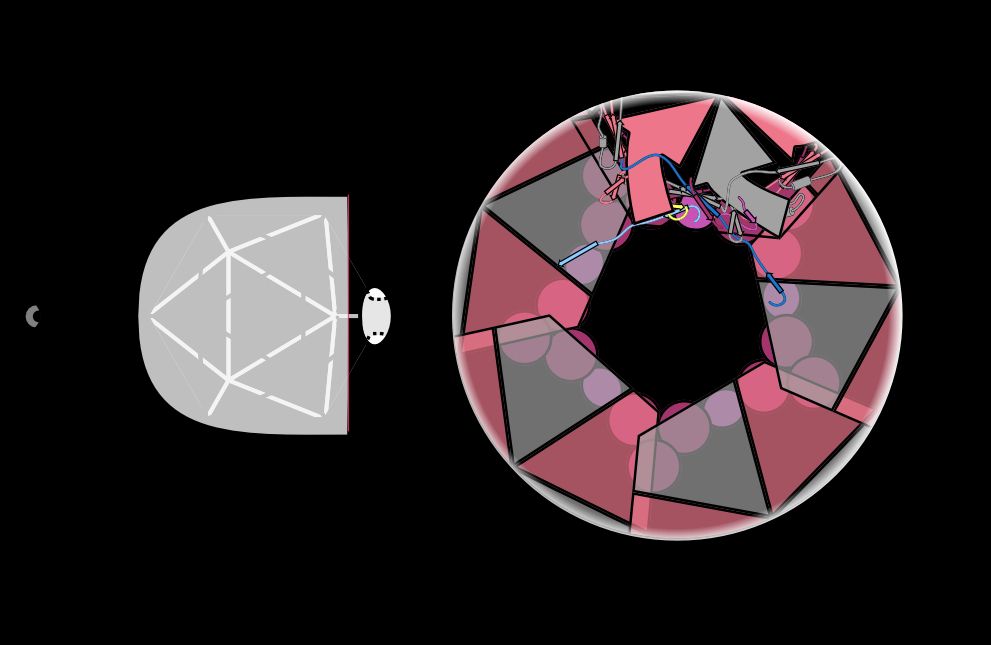

Instead of beefing up electrostatic or hydrophobic interactions between these proteins, the phage intertwines different subunits to create topological linkages. This is the same strategy used to make a wicker basket strong! /6
18.04.2025 14:15 — 👍 9 🔁 2 💬 1 📌 0

a man in a suit and tie is dancing .
ALT: a man in a suit and tie is dancing .
If this aperture were to dilate, it would allow the genome to explode out of the capsid and kill the phage! So how does the phage hold this aperture together at high temperatures, with tons of DNA pressing against it? /5
18.04.2025 14:14 — 👍 3 🔁 0 💬 1 📌 0

With 105 protein chains and 5 DNA segments, across 4 different symmetries, this is the largest structure the Kelch Lab has ever built (2.4 MDa)! The proteins in pinks/reds are part of the capsid shell, and they form an aperture that the rest of the Neck sits in. /4
18.04.2025 14:13 — 👍 7 🔁 0 💬 1 📌 0

Postdoc @emma.sedivy.bsky.social determined the structure of the Neck complex that joins the capsid head to the tail. We only knew the identity of 4 out of 8 of the proteins in the Neck, but we were able to identify the remaining 4 using ModelAngelo by @kjamali.bsky.social. /3
18.04.2025 14:12 — 👍 5 🔁 1 💬 1 📌 0

This phage not only lives in hot springs but also has the longest tail of any known phage. Here you see 3 viruses infecting their host, T. thermophilus. One has released its genome into the host, but two still have DNA-filled heads. /2
18.04.2025 14:10 — 👍 9 🔁 1 💬 1 📌 0
Meme describing types of headaches, with the most all encompassing headache being the stress of holding copious amounts of DNA in a phage head.
Did you know that there is so much DNA packed inside a phage capsid that the pressure is 10X higher than in a bottle of champagne? In our latest preprint, we wondered how that is contained in a phage that lives at extremely high temperatures. /1
www.biorxiv.org/content/10.1...
18.04.2025 14:09 — 👍 92 🔁 25 💬 6 📌 10
Virus origins/diversity/evolution; mobilome; Archaea.
Head of the Cell Biology and Virology of Archaea Unit at Institut Pasteur:
https://research.pasteur.fr/en/team/archaeal-virology/
News from the Sternberg lab at Columbia University, Howard Hughes Medical Institute.
Posts are from lab members and not Samuel Sternberg unless signed SHS. Posts represent personal views only.
Visit us at www.sternberglab.org
Phage scientist @KULeuven in Belgium | Science communicator | Phage therapy advocate for patients
Website motherofphage.com
Dad, Teacher, Artist, Gardener and Scientist.
Change is the only permanent thing in the world. This moment will pass. It has to.
Ps.
All I post is my personal opinion
Big phan of physics & phages | cryo-EM, phage engineering, a little immunology | e-bike enthusiast
CWRU Physics Assistant Professor
Cleveland kid.
Protein chemist, computational biologist, and general data enthusiast. Big fan of good food, sports, travel, and books. gbedwell.github.io
Chemical biologist passionate about post-translational modifications (PTMs) and a keen interest in drug development. Professor at OHSU leading a team dedicated to unraveling the mysteries of PARPs and ADP-ribosylation.
Graduate Student in the Maxwell Lab (Biochemistry, University of Toronto). Studying bacteria-phage interactions in Pseudomonas aeruginosa.
Phage Biology, Bacterial defense systems, CRISPR-Cas, Microbial communities, Mucus interactions
http://www.fnobregalab.org
http://www.klebphacol.org
http://www.phage-collection.org
HKer, nidovirologist. Revstar & MayaKuro fangirl. 一个健康的社會不該只有一種聲音. 解散警隊. ★ she/佢 ★
Microbiologist & phage phanatic! Enthusiastic explorer of microbial genomes.
PI at the Wadsworth Center, New York State Department of Health
Asst Prof @albany.edu
Currently obsessed with plasmid-dependent phages
www.wadsworth.org/research/laboratories/owen
American Cancer Society Postdoctoral fellow and structural biologist in the @briankelch.bsky.social lab at UMass Chan Medical school. I study ATPases and DNA replication/repair. Phage and MD simulations were my first scientific love. he/him/his
CryoEM, membrane proteins and whatnot
A leading life science journal that champions high-impact research across all disciplines—from molecules to ecosystems. We offer innovative formats and collaborative editorial support to ensure your work achieves its full scientific impact.
Scientist, Educator and #Myeloma Patient Advocate | Mom, wife, friend, avid reader & stress-relieving crocheter | Working daily to love my neighbor and show that basic science saves lives.
Our lab studies ssDNA gaps as the cause of BRCAness and investigates how these gaps create a therapeutic window for sensitizing cancer cells to anti-cancer therapies.
The Hedglin lab in the department of Chemistry at the Pennsylvania State University utilizes chemical, biochemical, and biophysical approaches to study the mechanisms of human genome integrity maintenance.
Neurogeneticist, transposonophile, drosophilophile, eonophile, foodie, husband, father, kayaker, dog in training, #BLM, anti fascist. 🛑 the GENOCIDE! Free Palestine 🇵🇸!
Editor in Chief of the #NonProfit, #OpenAccess journal @plosbiology.org Former Chief Editor of Nature Microbiology.
#Virologist. #Feminist. #Spaniard in the UK. #Galician. #European always.
Views my own.
https://orcid.org/0000-0002-3666-5683






























