SAVE THE DATE!
The EMBO workshop "Ubiquitin and Ubiquitin-like proteins in Health and Disease" will be on September 2026.
Exciting line up of talks and an amazing location!
Organised by @kulathu.bsky.social, @merbllab.bsky.social, @simonapolo.bsky.social, Claudio Joazeiro.
Registration open soon.
08.12.2025 13:16 — 👍 16 🔁 14 💬 0 📌 0

An Asgard archaeon with internal membrane compartments
Brilliant study led by @fmacleod.bsky.social and Andriko von Kügelgen. Tight collaboration with @buzzbaum.bsky.social and lab. Congrats to all authors!
www.biorxiv.org/content/10.1...
07.11.2025 10:44 — 👍 384 🔁 170 💬 10 📌 22

📢 Open Call! The Max Perutz Labs invite applications for a Full Professorship in Integrative Structure Biology with a focus on in situ structural biology using cryo-electron tomography (cryo-ET) and related methods. More details ➡️ tinyurl.com/brswbymu
31.10.2025 09:24 — 👍 27 🔁 44 💬 0 📌 2
Structure and substrate recognition by the Twin-arginine translocation (Tat) pathway core complex https://www.biorxiv.org/content/10.1101/2025.09.18.677151v1
19.09.2025 03:46 — 👍 8 🔁 4 💬 0 📌 0
We have an open post-doc position in my group to study mRNA cleavage and polyadenylation using biochemical reconstitution and cryoEM.
Please get in touch if you are interested in joining this amazing team! 🔬🧬🤩
#RNA #cryoEM
27.09.2025 11:06 — 👍 58 🔁 52 💬 0 📌 1
I am excited to share our new preprint on the CAGE complex, a mysterious hollow protein complex that I first saw years ago while surveying Tetrahymena ciliary lysate www.biorxiv.org/content/10.1... #cilia #protistsonsky 🧬🧪
23.09.2025 17:08 — 👍 167 🔁 54 💬 7 📌 13
Laura Lorenzo Orts
IMB Mainz
I am excited to announce that I will be moving to IMB Mainz next year! The Winter call for the IPP PhD program is now open; if you are interested in maternal #mRNA regulation and #translation in early vertebrate development, please apply! Deadline: 16 October.
More info: www.imb.de/students-pos...
15.09.2025 12:13 — 👍 66 🔁 25 💬 3 📌 3

Inside the cell’s recycling hub: unveiling the architecture of UBR4
Researchers from the lab of Tim Clausen and collaborators have unveiled the three-dimensional structure of UBR4, a giant protein complex that safeguards cells by targeting defective proteins for destr...
Researchers around @clausenlab.bsky.social & @dangrabarczyk.bsky.social have unveiled the three-dimensional structure of UBR4, a giant protein complex that safeguards cells by targeting defective proteins for destruction—offering fresh insights into health & disease. www.science.org/doi/10.1126/...
28.08.2025 18:28 — 👍 36 🔁 11 💬 2 📌 0
We had great help from Hyun Kyu Song's lab who got crystal structures of substrate complexes, as well as our ongoing collaboration with the Manu Hegde lab. Also thanks to the efficient review process which really pushed us to improve the paper.
28.08.2025 19:02 — 👍 0 🔁 0 💬 0 📌 0
Glad to share the final version of our story about the UBR4 complex, an E4 ligase protein quality control hub @science.org. Now with more cryo-EM structures and a deeper dive into substrate recognition, especially escaped mitochondrial proteins @clausenlab.bsky.social www.science.org/doi/10.1126/...
28.08.2025 18:54 — 👍 80 🔁 30 💬 7 📌 1

ZNFX1 compacts and tags RNA to keep immunity in check
Tim Clausen’s lab at the IMP and collaborators have uncovered how the ancient immune protein ZNFX1 enables cells to walk the fine line between fighting infection and avoiding autoimmune damage. The te...
Scientists uncovered how the immune protein ZNFX1 enables cells to walk the fine line between fighting infection & avoiding autoimmune damage. ZNFX1 can ubiquitinate & compact host & viral RNA into molecular condensates, extending ubiquitin biology to RNA itself. More: www.imp.ac.at/news/article...
28.08.2025 07:33 — 👍 35 🔁 8 💬 2 📌 2
Research Assistant/Associate
Research Assistant/Associate COLLEGE OF MVLSSchool of Infection & ImmunityResearch and TeachingGRADE 6/7 Job PurposeThe post holder will join the School of Infection & Immunity, MRC-Univers...
Opening for a biochemist to help us in the characterisation of novel ubiquitin-based antivirals. Diverse backgrounds welcomed; a keen sense of adventure is a must 😁
Details and criteria are below; please get in touch with any questions.
www.jobs.gla.ac.uk/job/research...
25.08.2025 12:34 — 👍 3 🔁 4 💬 0 📌 0
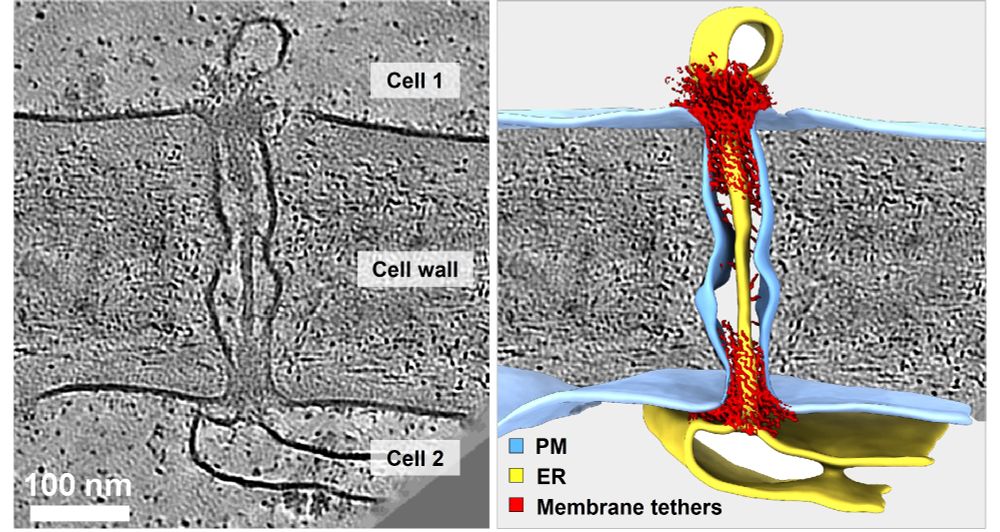
New preprint: In situ Architecture of #Plasmodesmata.
Using #cryoET, #AlphaFold & proteomics, we uncover the native organization of plant intercellular nanopores.
🔗 doi.org/10.1101/2025...
🧪🧵1/n
#teamtomo #PlantScience #Physcomitrium #Arabidopsis #cryoEM
26.07.2025 15:38 — 👍 196 🔁 71 💬 9 📌 8

🎉 Congratulations to our Daniel Grabarczyk, research associate in the Clausen lab, who has been awarded a prestigious FWF Principal Investigator Project Grant to support his research into the molecular mechanisms of innate immunity against viruses.
Read more: www.imp.ac.at/news/article...
09.07.2025 08:53 — 👍 27 🔁 3 💬 2 📌 0
Thrilled to share the structure of dimerised human PINK1 docked to an endogenous translocase array on the mitochondrial surface, composed of two TOM complexes, bridged by a VDAC2 dimer! Published today in Science www.science.org/doi/10.1126/...
@wehi-research.bsky.social @komanderlab.bsky.social
13.03.2025 19:19 — 👍 167 🔁 56 💬 8 📌 6
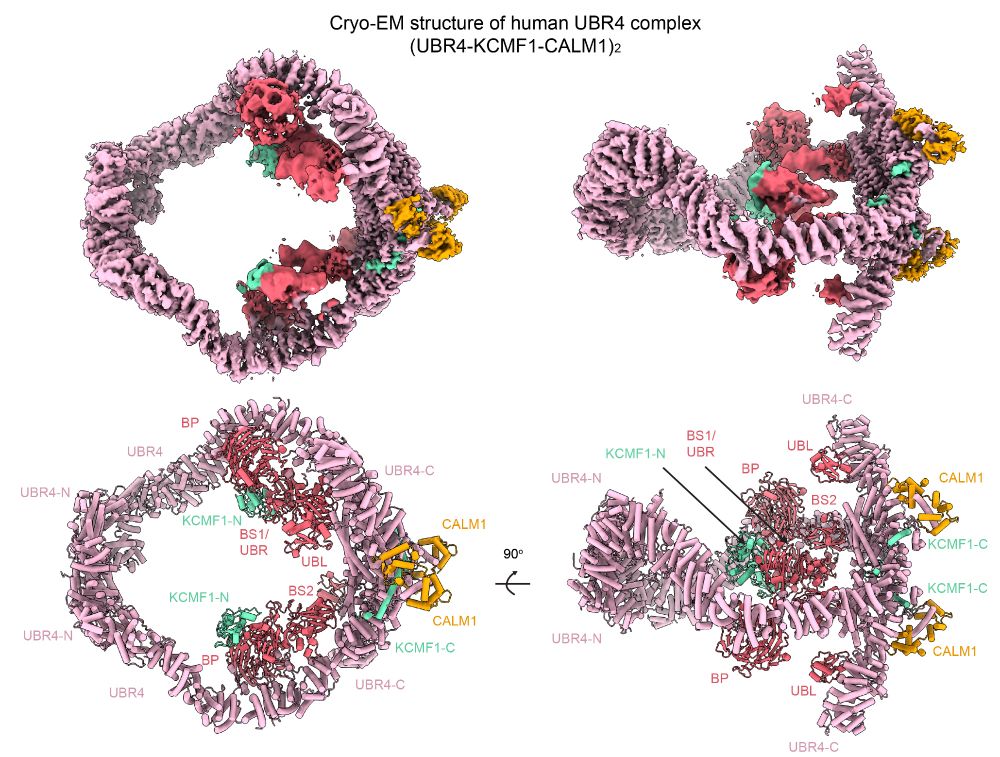
Architecture of the 1.3 MDa complex of human UBR4 (2 x UBR4/KCMF1/CALM1). Picture shows top and side views of the cryo-EM density map and the modeled structure, colored by protein components and domains.
🎄 xmas preprint 🎄 we are excited to share our cryo-EM structure of UBR4 in complex with KCMF1 and CALM1. the PQC ligase forms a massive ubiquitination arena, primed to amplify ubiquitin chains (E4 activity) and boost degradation of defective proteins. www.biorxiv.org/content/10.1...
21.12.2024 10:46 — 👍 132 🔁 37 💬 3 📌 4
Happy to share our UBR4 structures. Check out the preprint to see how this giant E4 ligase complex selects substrates for ubiquitin chain extension. We also look at conservation and adaptations across humans, nematodes and plants.
www.biorxiv.org/content/10.1...
@clausenlab.bsky.social
21.12.2024 09:13 — 👍 66 🔁 16 💬 2 📌 1
At the Department of Biochemistry, University of Oxford, UK. Structural biology. Ubiquitination pathways. elliottlab.web.ox.ac.uk
Postdoc @UoD @CeTPD with Alessio Ciulli
Structural & chemical biology in Targeted Protein Degradation
Postdoctoral Researcher @NynkeDekkerLab @UniofOxford @KavliOxford Ph.D. @MRC_LMB @Yeeles_Lab @Cambridge_Uni / DNA replication &replication stress enthusiast 🧬
Structural biologist working to understand DNA replication at the MRC-LMB
Cell biologist and a cat person. I mainly study CDK8/19, cell cycle regulation, and new anti-cancer drug candidates.
https://orcid.org/0000-0002-9080-5683
PhD Student in Sascha Martens Lab @MaxPerutzLabs studying how cells remove protein aggregates using autophagy.
Postdoc in the lab of Carina de Oliveira Mann
TUM, Munich, Germany, interested in pathogens and innate immunity
https://www.bio.nat.tum.de/en/cryoem/home/
MSCA Postdoc fellow, Van Breusegem Lab, Oxidative Stress Signalling. VIB-UGent Center for Plant Systems Biology
Postdoc, antisense oligomers, computational (micro)biology, antibiotics, 🦠bacteria🦠, microbiome, immunology, AMR, global health, statistics.
he/him
https://linkedin.com/in/jakob-jung/
https://github.com/jakobjung
https://mstdn.science/@jakobjung
mRNA & cryo-EM enthusiasts at IMP Vienna. Posts are by lab members.
PhD student at the Brennecke and Plaschka labs, Vienna.
Interested in RNA silencing
Amaro Lab (UC San Diego), PhD student Biochemistry and Molecular Biophysics | Computational Biology
Assistant Professor @ ISTA
Using cryo-EM to understand how bacteria defend themselves
https://bravo-lab.org/
PhD student | MRC-UofG Centre for Virus Research 🦠🏴
E3 ligases in innate immunity
(she/her)
Investigating kinase-dependent regulation of DNA replication and repair
Postdoc working on E3 ligases in the context of viral infections. University of Glasgow, CVR based 🧬
Lead Editor: #CRBIOTECH & #ExplorDHT | PI: PaDiH & LBI-DHPS | Prof of IGAB-PAS | Leader: #DHPSP & #INPST | Expert Consultant in Biotech & Science Communication
🔗Web: https://digitalpatientsafety.com/atanas-g-atanasov/
I am an Assistant Professor of Cancer Biology at Loyola University Chicago Stritch School of Medicine (www.fanninglab.com). The Fanning lab studies how allostery governs estrogen receptor-dependent transcriptional programs in breast cancer.
Convergence Science PhD student at the Institute of Cancer Research and Imperial College London.
A structural biologist for understanding protein degradation mechanism