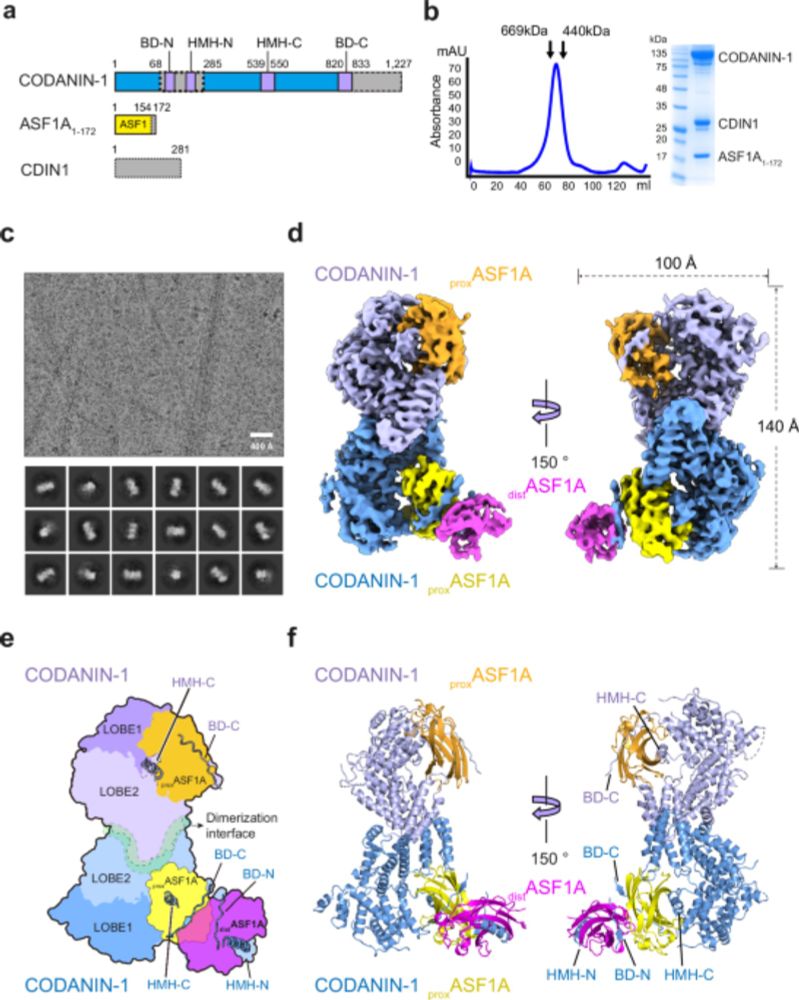
Cells control the supply of new histones to chromatin by blocking histone chaperone function - see here how Codanin-1 sequesters 4 ASF1 molecules.
23.03.2025 12:04 — 👍 24 🔁 7 💬 0 📌 0
Brilliant summaries from our wonderful collaborator @grothlab.bsky.social . I'm always grateful for your enthusiasm, and I hope the next chapter of our story!
07.03.2025 16:30 — 👍 2 🔁 0 💬 1 📌 0
Furthermore, our structural and biochemical analyses, combined with in-cell studies, demonstrate that the dual-binding mode of CODANIN-1 and ASF1 is essential for the efficient cytoplasmic sequestration of ASF1.
07.03.2025 16:21 — 👍 0 🔁 0 💬 1 📌 0
This structural feature provides direct evidence that CODANIN-1 competes with histone H3/H4 for ASF1 binding. Such competition suggests that CODANIN-1 functions as a negative regulator of DNA replication by interfering with the formation of the histone H3/H4–ASF1 complex.
07.03.2025 16:21 — 👍 0 🔁 0 💬 1 📌 0
Interestingly, in addition to the previously known ASF1 binding motif of CODANIN-1, we identified novel binding motifs. Among them, the discovery of binding motifs that mimic the histone H3 helix was particularly striking. We named these helices the histone mimic helices (HMHs).
07.03.2025 16:21 — 👍 0 🔁 0 💬 1 📌 0
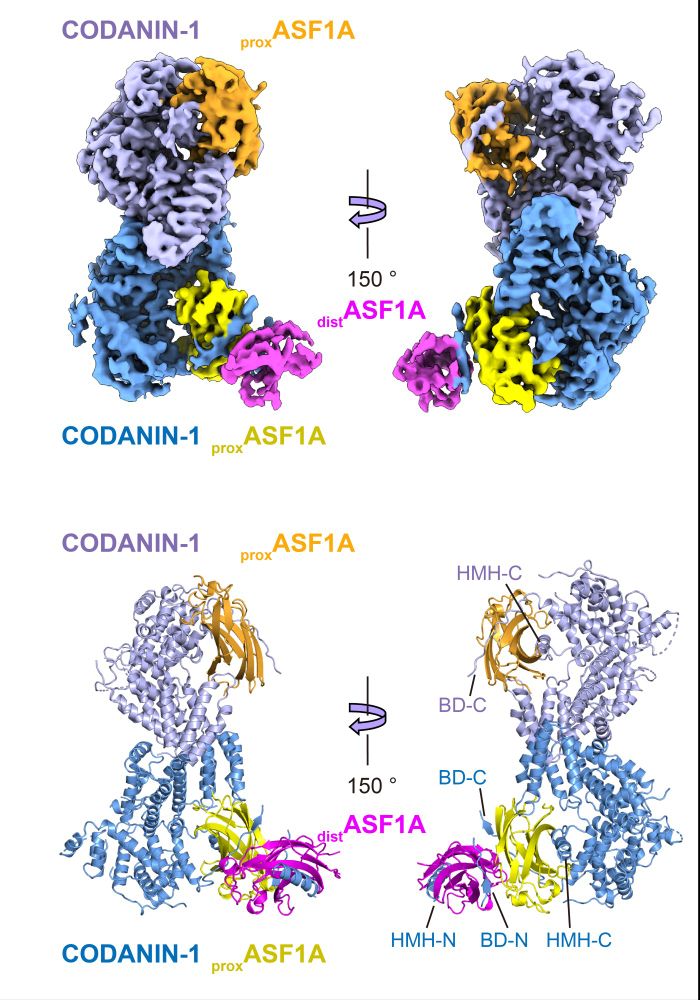
Our study reveals the molecular mechanism of ASF1 cytoplasmic sequestration by CODANIN-1 based on the cryo-EM structure of the CODANIN-1_ASF1A complex. In our structure, CODANIN-1 exists as a dimer, with each CODANIN-1 monomer binding to two ASF1A molecules to form the complex.
07.03.2025 16:21 — 👍 0 🔁 0 💬 1 📌 0
cellarchlab.com Univ. of Basel Biozentrum🇨🇭. Exploring molecular architecture inside cells with #CryoEM #TeamTomo. Plants and algae in a changing climate. ❄🔬 OF 🌿 4THE 🌍!
In Almouzni Lab at Institut Curie, a bioinformatician among H3 histone variants afficionados, exploring their role in shaping chromatin in pediatric brain cancers.
Using my cells to study cells 🧬 ~ Postdoc: histone mark inheritance @Grothlab ~ PhD: PP2A control of DNA replication @Zegermanlab
Asst. Prof. at WashU School of Medicine; biophysics/biochem/evolution of intrinsically disordered proteins. How does nature encode function without a stable structure? We work in vivo / in vitro / in silico. He/him.
https://www.holehouselab.com/
Our group aims to elucidate the molecular basis of cytoplasmic organization.
stress granules I biomolecular condensates I phase separation
https://tu-dresden.de/cmcb/biotec/forschungsgruppen/alberti
Science and fun from the lab of Tony Hyman at the MPI-CBG @mpicbg.bsky.social. Tweets by Hymanlab members.
https://hymanlab.org/
PI @ Gladstone Institutes & UCSF. Molecular technologies & the genomics / molecular biology / biochemistry of gene regulation. Views here mine & do not represent those of my affiliated institutions.
Assistant Professor @Cornell University
- Germline gene regulation, gene silencing, chromatin
- jongminkimlab.org
Salipro Biotech AB is a privately held biotech company focused on unlocking challenging drug targets for the development of next-generation therapeutics. The company is headquartered in Stockholm, Sweden.
The Epigenetics Podcast of @activemotifusa.bsky.social, hosted by @johndillinger15.bsky.social
https://activemotif.com/podcasts
#epigenetics #chromatin #podcast
Structural biologist | CryoCloud 🔬☁️ | Posts about #cryoEM, some running and cats | Views my own | She/her
Revealing the hidden world of shape shifting enzymes using Cryo-EM. Senior research associate at Newcastle University, UK. Currently working as part of Recon4IMD https://www.recon4imd.org Views are my own.
Post doc and manga lover, I will be sharing science-related stuff and manga reads.
Husband, Father and grandfather, Datahound, Dog lover, Fan of Celtic music, Former NIGMS director, Former EiC of Science magazine, Stand Up for Science advisor, Pittsburgh, PA
NIH Dashboard: https://jeremymberg.github.io/jeremyberg.github.io/index.html
Assistant Professor of Cell Biology at Harvard Medical School | Nexus of chromatin, transcription, replication, and epigenetics. farnunglab.com
Joint Head of Structural Studies at @mrclmb.bsky.social. Develops & uses #cryoEM to study amyloids in neurodegeneration. #tau, #alphasynuclein, #opensoftware, #RELION. All opinions my own.
Chromatin and cryoEM afficionada. Still a fan of crystallography. Avid Colorado hiker. Will call out ugly nucleosome cartoons. Come for the science, stay for the mountain pictures and snark. Opinions and snark are my own.
The Anja Groth group in Copenhagen studies chromatin replication, epigenetic memory and (epi)genome stability in the context of mitotic cell division
Group leader at the Danish Cancer Institute and Professor at University of Copenhagen #NNFCPR. Interested in epigenetics, genome maintenance, aging & cancer, creativity & innovation, society in general. Opinions are my own.